Human endoplasmic reticulum mannosidase I is subject to regulated proteolysis - PubMed (original) (raw)
Human endoplasmic reticulum mannosidase I is subject to regulated proteolysis
Ying Wu et al. J Biol Chem. 2007.
Abstract
In the early secretory pathway, opportunistic cleavage of asparagine-linked oligosaccharides by endoplasmic reticulum (ER) mannosidase I targets misfolded glycoproteins for dislocation into the cytosol and destruction by 26 S proteasomes. The low basal concentration of the glycosidase is believed to coordinate the glycan cleavage with prolonged conformation-based ER retention, ensuring that terminally misfolded glycoproteins are preferentially targeted for destruction. Herein the intracellular fate of human ER mannosidase I was monitored to determine whether a post-translational process might contribute to the regulation of its intracellular concentration. The transiently expressed recombinant human glycosidase was subject to rapid intracellular turnover in mouse hepatoma cells, as was the endogenous mouse ortholog. Incubation with either chloroquine or leupeptin, but not lactacystin, led to intracellular stabilization, implicating the involvement of lysosomal acid hydrolases. Inhibition of protein synthesis with cycloheximide led to intracellular depletion of the glycosidase and concomitant ablation of asparagine-linked glycoprotein degradation, confirming the physiologic relevance of the destabilization process. Metabolic incorporation of radiolabeled phosphate, detection by anti-phosphoserine antiserum, and the stabilizing effect of general serine kinase inhibition implied that ER mannosidase I is subjected to regulated proteolysis. Stabilization in response to genetically engineered removal of the amino-terminal cytoplasmic tail, a postulated regulatory domain, and colocalization of green fluorescent protein fusion proteins with Lamp1 provided two additional lines of evidence to support the hypothesis. A model is proposed in which proteolytically driven checkpoint control of ER mannosidase I contributes to the establishment of an equitable glycoprotein quality control standard by which the efficiency of asparagine-linked glycoprotein conformational maturation is measured.
Figures
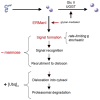
FIGURE 1. Conformational maturation and intracellular disposal of newly synthesized asparagine-linked glycoproteins represent a conformation-based branch point in the early secretory pathway
Modification of asparagine-linked oligosaccharides by glucosidase II (GlcII) and UDP-Glc:glycoprotein glucosyltransferase (UGGT) facilitates rounds of physical engagement with glycoprotein folding machinery. Conformational maturation (solid sphere) precedes productive transport and deployment. In contrast, nonnative protein structure, in combination with glycans cleaved by ERManI, generates a proposed lumenal bipartite signal for recruitment to the ER dislocon. The low basal ERManI concentration underlies a quantitative trait by which asparagine-linked glycoproteins are selected for destruction on the basis of inefficient conformational. The latter parameter is designated by opportunistic cleavage of asparagine-linked oligosaccharides in response to prolonged conformation-based retention in the ER. Dislocation into the cytosol and elimination by 26 S proteasomes are mediated by polyubiquitination.
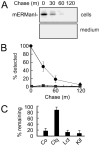
FIGURE 2. Intracellular turnover of transfected (transf.) recombinant human ER mannosidase I
A, detection by ECL Western blotting of transiently expressed recombinant human ER mannosidase I. B, pulse-chase metabolic radiolabeling with [35S]Met and selective immunoprecipitation of recombinant human ER mannosidase I. C, results from B are depicted as a graph. All of the experiments were initiated ~48–72 h post-transfection. Specific antiserum (“Experimental Procedures”) resulted in the detection of the recombinant protein in Nonidet P-40 cell lysates (cells) and tissue culture medium (media). Duration of the metabolic pulse in B was 10 min.
FIGURE 3. Pharmacologic inhibition of recombinant human ER mannosidase I turnover
A, pulse-chase metabolic radiolabeling with [35S]Met and selective immunoprecipitation of recombinant human ER mannosidase I. Co, normal medium; Lct, medium with the addition of lactacystin; Clq, medium with the addition of chloroquine; Kif, medium with the addition of kifunensine (“Experimental Procedures”). B, same as above, but with the addition of leupeptin (Leup) (“Experimental Procedures”). Duration of the metabolic pulse in B was 10 min.
FIGURE 4. Endogenous mouse ER mannosidase I is subject to rapid intracellular turnover
A, pulse-chase metabolic radiolabeling with [35S]Met and selective immunoprecipitation of endogenous mouse ER mannosidase I from Nonidet P-40 cell lysates (cells) and tissue culture medium (medium). Duration of the metabolic pulse was 10 min. B, results from A are depicted as a graph. C, the effects of chloroquine (Clq), lactacystin (Lct), and kifunensine (Kif) on the intracellular fate of endogenous mouse ER mannosidase I, relative to control (Co), are depicted as a bar graph. All of the experiments were initiated ~48–72 h post-transfection. Specific antiserum (“Experimental Procedures”) resulted in the detection of the recombinant protein in Nonidet P-40 cell lysates and tissue culture medium. Duration of the metabolic pulse in B was 10 min.
FIGURE 5. Intracellular depletion of ER mannosidase I and concomitant inhibition of GERAD in response to a 1-h incubation in medium containing cycloheximide
A, detection of endogenous mERManI and grp78/BiP by ECL Western blotting from Nonidet P-40 cell lysates under control conditions (Co) or following incubation with cycloheximide (Chx). Protein G used without antiserum (pG). B, pulse-chase metabolic radiolabeling with [35S]Met and immunoprecipitation of _α_1-antitrypsin variant null(Hong Kong) (NHK) in cell line H1A/N13 (“Experimental Procedures”) under control conditions (Co) or following treatment with cycloheximide (Chx) or kifunensine (Kif). Duration of the metabolic pulse in B was 10 min.
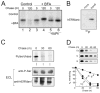
FIGURE 6. Detectable phosphorylation of recombinant human ER man-nosidase I and arrested intracellular turnover in response to selective inhibition of serine kinase activity
A, pulse-chase metabolic radiolabeling with [35S]Met and selective immunoprecipitation of recombinant human ER mannosidase I from Nonidet P-40 cell lysates under normal conditions (Control) or with the addition of brefeldin A (+BFA). Samples treated with potato acid phosphatase (+AchP) are shown. B, selective immunoprecipitation of recombinant human ER mannosidase I from mock transfected (mock) and transfected (transf) Hepa1a Nonidet P-40 cell lysates followed a 3-h incubation with phosphate-free growth medium supplemented with radiolabeled inorganic phosphate (32P). C, metabolic pulse-chase radiolabeling with [35S]methionine (top panel), ECL Western blotting with an anti-phospho-Ser antibody of human ER mannosidase I immunoprecipitates from mock transfected (mock) and transfected (transf) Hepa1a cells (middle panel), and ECL Western blotting for human ERManI (bottom panel). D, pulse-chase metabolic radiolabeling with [35S]Met and selective immunoprecipitation of recombinant human ER mannosidase I under control (Co) conditions or following treatment with staurosporine (S) or genistein (G). The quantified results are shown (graph). Duration of the metabolic pulses in A and D was 10 min.
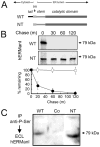
FIGURE 7. Arrested intracellular turnover in response to genetically engineered removal of the amino-terminal cytoplasmic tail
A, relative topology in the ER membrane of the domains for wild type (WT) human ER mannosidase I, including the amino-terminal cytoplasmic tail (tail), transmembrane domain (tm), and lumenal domains (stem and catalytic domain) before (WT) and after genetic-engineered removal of the amino-terminal cytoplasmic tail (NT). B, pulse-chase metabolic radiolabeling with [35S]Met (10-min pulse) and selective immunoprecipitation from Nonidet P-40 cell lysates following transient expression of the wild type recombinant human ER mannosidase (WT) and following genetic-engineered removal of the amino-terminal cytoplasmic tail (NT). In the lower panel, the results are presented as a graph. C, Nonidet P-40 lysates from untransfected cells (Co), and cells transfected with either WT and NT were immunoprecipitated (IP) with anti-phospho-antiserum and fractionated by SDS-PAGE, and human ERManI was detected by ECL Western blotting.
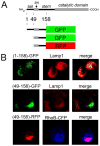
FIGURE 8. Colocalization with lysosomes and contribution of the amino-terminal cytoplasmic tail
A, schematic diagram of hERManI domain structure and design of fluorescent fusion proteins (“Experimental Procedures”). B, confocal microscopy 72 h post-transfection of Hepa1a cells was used to detect and localize the fluorescent fusion proteins shown in A. Endogenous Lamp1 was used as a marker for lysosomes. Cotransfected RhoB-CFP served a fluorescent marker for endosomes.
Similar articles
- Elucidation of the molecular logic by which misfolded alpha 1-antitrypsin is preferentially selected for degradation.
Wu Y, Swulius MT, Moremen KW, Sifers RN. Wu Y, et al. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8229-34. doi: 10.1073/pnas.1430537100. Epub 2003 Jun 18. Proc Natl Acad Sci U S A. 2003. PMID: 12815101 Free PMC article. - The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation.
Banerjee S, Vishwanath P, Cui J, Kelleher DJ, Gilmore R, Robbins PW, Samuelson J. Banerjee S, et al. Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11676-81. doi: 10.1073/pnas.0704862104. Epub 2007 Jul 2. Proc Natl Acad Sci U S A. 2007. PMID: 17606910 Free PMC article. - Role of N-linked polymannose oligosaccharides in targeting glycoproteins for endoplasmic reticulum-associated degradation.
Spiro RG. Spiro RG. Cell Mol Life Sci. 2004 May;61(9):1025-41. doi: 10.1007/s00018-004-4037-8. Cell Mol Life Sci. 2004. PMID: 15112051 Free PMC article. Review. - Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation.
Parodi AJ. Parodi AJ. Biochem J. 2000 May 15;348 Pt 1(Pt 1):1-13. Biochem J. 2000. PMID: 10794707 Free PMC article. Review.
Cited by
- Large protein complexes retained in the ER are dislocated by non-COPII vesicles and degraded by selective autophagy.
Le Fourn V, Park S, Jang I, Gaplovska-Kysela K, Guhl B, Lee Y, Cho JW, Zuber C, Roth J. Le Fourn V, et al. Cell Mol Life Sci. 2013 Jun;70(11):1985-2002. doi: 10.1007/s00018-012-1236-6. Epub 2013 Jan 22. Cell Mol Life Sci. 2013. PMID: 23338832 Free PMC article. - Proteostasis: a new therapeutic paradigm for pulmonary disease.
Bouchecareilh M, Balch WE. Bouchecareilh M, et al. Proc Am Thorac Soc. 2011 May;8(2):189-95. doi: 10.1513/pats.201008-055MS. Proc Am Thorac Soc. 2011. PMID: 21543800 Free PMC article. - Arms Race between Enveloped Viruses and the Host ERAD Machinery.
Frabutt DA, Zheng YH. Frabutt DA, et al. Viruses. 2016 Sep 19;8(9):255. doi: 10.3390/v8090255. Viruses. 2016. PMID: 27657106 Free PMC article. Review. - ERManI is a target of miR-125b and promotes transformation phenotypes in hepatocellular carcinoma (HCC).
Pan S, Cheng X, Chen H, Castro PD, Ittmann MM, Hutson AW, Zapata SK, Sifers RN. Pan S, et al. PLoS One. 2013 Aug 5;8(8):e72829. doi: 10.1371/journal.pone.0072829. Print 2013. PLoS One. 2013. PMID: 23940818 Free PMC article. - Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes.
Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Kim PK, et al. Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20567-74. doi: 10.1073/pnas.0810611105. Epub 2008 Dec 12. Proc Natl Acad Sci U S A. 2008. PMID: 19074260 Free PMC article.
References
- Gerstein M, Lan N, Jansen R. Science. 2002;295:284–287. - PubMed
- Cabral CM, Liu Y, Sifers RN. Trends Biochem Sci. 2001;26:619–624. - PubMed
- Ellgaard L, Helenius A. Curr Opin Cell Biol. 2001;13:431–437. - PubMed
- McCracken AA, Brodsky JL. Bioessays. 2003;25:868–877. - PubMed
- Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, Sommer T. Nat Cell Biol. 2002;4:134–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL062553/HL/NHLBI NIH HHS/United States
- R01 DK075322/DK/NIDDK NIH HHS/United States
- U01 CA091295/CA/NCI NIH HHS/United States
- GM47533/GM/NIGMS NIH HHS/United States
- DK064232/DK/NIDDK NIH HHS/United States
- P41 RR005351/RR/NCRR NIH HHS/United States
- R01 DK064232/DK/NIDDK NIH HHS/United States
- RR005351/RR/NCRR NIH HHS/United States
- R01 GM047533/GM/NIGMS NIH HHS/United States
- HL62553/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous