An SCN9A channelopathy causes congenital inability to experience pain - PubMed (original) (raw)
. 2006 Dec 14;444(7121):894-8.
doi: 10.1038/nature05413.
Frank Reimann, Adeline K Nicholas, Gemma Thornton, Emma Roberts, Kelly Springell, Gulshan Karbani, Hussain Jafri, Jovaria Mannan, Yasmin Raashid, Lihadh Al-Gazali, Henan Hamamy, Enza Maria Valente, Shaun Gorman, Richard Williams, Duncan P McHale, John N Wood, Fiona M Gribble, C Geoffrey Woods
Affiliations
- PMID: 17167479
- PMCID: PMC7212082
- DOI: 10.1038/nature05413
An SCN9A channelopathy causes congenital inability to experience pain
James J Cox et al. Nature. 2006.
Abstract
The complete inability to sense pain in an otherwise healthy individual is a very rare phenotype. In three consanguineous families from northern Pakistan, we mapped the condition as an autosomal-recessive trait to chromosome 2q24.3. This region contains the gene SCN9A, encoding the alpha-subunit of the voltage-gated sodium channel, Na(v)1.7, which is strongly expressed in nociceptive neurons. Sequence analysis of SCN9A in affected individuals revealed three distinct homozygous nonsense mutations (S459X, I767X and W897X). We show that these mutations cause loss of function of Na(v)1.7 by co-expression of wild-type or mutant human Na(v)1.7 with sodium channel beta(1) and beta(2) subunits in HEK293 cells. In cells expressing mutant Na(v)1.7, the currents were no greater than background. Our data suggest that SCN9A is an essential and non-redundant requirement for nociception in humans. These findings should stimulate the search for novel analgesics that selectively target this sodium channel subunit.
Conflict of interest statement
The authors declare no competing financial interests. Correspondence and requests for materials should be addressed to C.G.W. (cw347@cam.ac.uk).
Figures
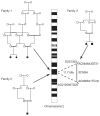
Fig. 1. The families used to map the locus for channelopathy-associated insensitivity to pain.
Autozygosity mapping in families 1 and 2 on the left of the diagram enabled the identification of a shared 20 cM homozygous region on chromosome 2q24 (there were no other significant homozygous regions detected). The two-point LOD score was 3.2 at θ = 5 0, for AC064843GT21 and AC092641TG18, but greater for more informative markers, see Supplementary Table 1. Family 3, on the right of the diagram, was used to refine this region to an 11.7-Mb shared homozygous region flanked by the heterozygous markers D2S1353 and AC012594TG25 and defined by the homozygous markers AC064843GT21 and AC092641TG18. Affected individuals are indicated with filled symbols.
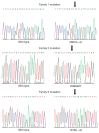
Fig. 2. Sequence chromatograms showing the mutations identified in families 1, 2 and 3.
The arrows indicate the site of the mutations.
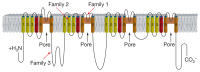
Fig. 3. Schematic representation of Nav1.7, the voltage-gated sodium channel _α_-subunit encoded by SCN9A, and the locations of the identified human mutations.
SCN9A encodes a plasma membrane protein: in the figure, the plasma membrane is shown in grey; the extracellular region is uppermost; and intracellular region below. Nav1.7 is predicted to fold into four similar domains with each domain comprising six α-helical transmembrane segments (labelled 1–6). Transmembrane segments 5 and 6 are the pore-lining segments and the voltage sensor is located in transmembrane segment 4 of each domain (depicted by a plus symbol). The red arrows indicate the location of the nonsense mutation in each family.
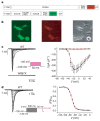
Fig. 4. Patch-clamping experiments to investigate the voltage-gated sodium channel activity of wild-type and truncated Nav1.7.
a, Constructs used in the patch-clamping experiments (see Supplementary Methods). We cloned wild-type SCN9A and then used site-directed mutagenesis to manufacture three further constructs each containing a family mutation. Each SCN9A construct was sequentially co-transfected with a plasmid containing the auxiliary sodium channel β1 and β2 subunits, and only cells clearly expressing DsRed2 (red fluorescence) and EGFP (green fluorescence) were measured for electrical activity. ECMV, encephalomyocarditis virus; IRES, internal ribosome entry site. b, A typical cell used in the patch-clamping experiments, showing both EGFP fluorescence (left) and DsRed2 fluorescence (middle), with the pipette attached in phase contrast (right). c, Left panel: initial current responses to 50-ms voltage steps of 5-mV increments between −70 and +40 mV from a holding potential of −100 mV, in a whole-cell voltage clamp recording applied at ∼0.5 Hz for a cell co-expressing wild-type (WT) Nav1.7 (top) or Nav1.7 W897X (bottom) with the β-subunits. The inset shows the voltage pulse protocol. Right panel: current–voltage relationship of the peak currents normalized for cell size (pA per pF) obtained using the experimental set-up shown on the left. Black squares, wild type (n = 13); red circles, I767X (n = 7); blue squares, W897X (n = 7); green diamonds, S459X (n = 5); white diamonds, β-subunits only (n = 5). The red line represents a fit of the wild-type data with a Boltzmann equation y = (_A_2 + (_A_1 − _A_2)/(1 + exp((_V_0.5 − x)/k)))(x − _V_rev), where _V_0.5 = 28.0 mV, k = 4.9 mV, _V_rev = 64 mV. d, Left panel: voltage dependence of the steady-state inactivation of wild-type Nav1.7 plus β-subunits was measured by holding the membrane potential for 500 ms at conditioning voltages from −120 to 0 mV (at 5-mV increments) before stepping to a test pulse at −10 mV for 50 ms. The inset shows the voltage pulse protocol, which was applied at 0.5 Hz. Only the current responses to the test pulse are shown. Right panel: the peak currents obtained as on the left were normalized to the maximum peak current, and plotted against the holding potential applied during the conditioning pulse. The red line represents a fit of the data with a Boltzmann equation y = (_A_1 − _A_2)/(1 + exp((x − _V_0.5)/k)) + _A_2, where _V_0.5 = −71 mV, k = 5.9 mV, n = 13. Error bars in c and d represent standard errors.
Comment in
- Neurobiology: a channel sets the gain on pain.
Waxman SG. Waxman SG. Nature. 2006 Dec 14;444(7121):831-2. doi: 10.1038/444831a. Nature. 2006. PMID: 17167466 No abstract available. - Potential downsides of perfect pain relief.
Mannes A, Iadarola M. Mannes A, et al. Nature. 2007 Mar 1;446(7131):24. doi: 10.1038/446024a. Nature. 2007. PMID: 17330023 No abstract available.
Similar articles
- A stop codon mutation in SCN9A causes lack of pain sensation.
Ahmad S, Dahllund L, Eriksson AB, Hellgren D, Karlsson U, Lund PE, Meijer IA, Meury L, Mills T, Moody A, Morinville A, Morten J, O'donnell D, Raynoschek C, Salter H, Rouleau GA, Krupp JJ. Ahmad S, et al. Hum Mol Genet. 2007 Sep 1;16(17):2114-21. doi: 10.1093/hmg/ddm160. Epub 2007 Jun 27. Hum Mol Genet. 2007. PMID: 17597096 - Congenital insensitivity to pain: novel SCN9A missense and in-frame deletion mutations.
Cox JJ, Sheynin J, Shorer Z, Reimann F, Nicholas AK, Zubovic L, Baralle M, Wraige E, Manor E, Levy J, Woods CG, Parvari R. Cox JJ, et al. Hum Mutat. 2010 Sep;31(9):E1670-86. doi: 10.1002/humu.21325. Hum Mutat. 2010. PMID: 20635406 Free PMC article. - Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders.
Drenth JP, Waxman SG. Drenth JP, et al. J Clin Invest. 2007 Dec;117(12):3603-9. doi: 10.1172/JCI33297. J Clin Invest. 2007. PMID: 18060017 Free PMC article. Review. - Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations.
Goldberg YP, MacFarlane J, MacDonald ML, Thompson J, Dube MP, Mattice M, Fraser R, Young C, Hossain S, Pape T, Payne B, Radomski C, Donaldson G, Ives E, Cox J, Younghusband HB, Green R, Duff A, Boltshauser E, Grinspan GA, Dimon JH, Sibley BG, Andria G, Toscano E, Kerdraon J, Bowsher D, Pimstone SN, Samuels ME, Sherrington R, Hayden MR. Goldberg YP, et al. Clin Genet. 2007 Apr;71(4):311-9. doi: 10.1111/j.1399-0004.2007.00790.x. Clin Genet. 2007. PMID: 17470132 - Sodium channelopathies and pain.
Lampert A, O'Reilly AO, Reeh P, Leffler A. Lampert A, et al. Pflugers Arch. 2010 Jul;460(2):249-63. doi: 10.1007/s00424-009-0779-3. Epub 2010 Jan 26. Pflugers Arch. 2010. PMID: 20101409 Review.
Cited by
- Genetics of disc-related disorders: current findings and lessons from other complex diseases.
Näkki A, Battié MC, Kaprio J. Näkki A, et al. Eur Spine J. 2014 Jun;23 Suppl 3:S354-63. doi: 10.1007/s00586-013-2878-2. Epub 2013 Jul 10. Eur Spine J. 2014. PMID: 23838702 Review. - The different mechanisms of peripheral and central TLR4 on chronic postsurgical pain in rats.
Han X, Shao J, Ren X, Li Y, Yu W, Lin C, Li L, Sun Y, Xu B, Luo H, Zhu C, Cao J, Li Z. Han X, et al. J Anat. 2021 Jul;239(1):111-124. doi: 10.1111/joa.13406. Epub 2021 Mar 17. J Anat. 2021. PMID: 33730389 Free PMC article. - Subtype-Selective Small Molecule Inhibitors Reveal a Fundamental Role for Nav1.7 in Nociceptor Electrogenesis, Axonal Conduction and Presynaptic Release.
Alexandrou AJ, Brown AR, Chapman ML, Estacion M, Turner J, Mis MA, Wilbrey A, Payne EC, Gutteridge A, Cox PJ, Doyle R, Printzenhoff D, Lin Z, Marron BE, West C, Swain NA, Storer RI, Stupple PA, Castle NA, Hounshell JA, Rivara M, Randall A, Dib-Hajj SD, Krafte D, Waxman SG, Patel MK, Butt RP, Stevens EB. Alexandrou AJ, et al. PLoS One. 2016 Apr 6;11(4):e0152405. doi: 10.1371/journal.pone.0152405. eCollection 2016. PLoS One. 2016. PMID: 27050761 Free PMC article. - SCN9A Variants May be Implicated in Neuropathic Pain Associated With Diabetic Peripheral Neuropathy and Pain Severity.
Li QS, Cheng P, Favis R, Wickenden A, Romano G, Wang H. Li QS, et al. Clin J Pain. 2015 Nov;31(11):976-82. doi: 10.1097/AJP.0000000000000205. Clin J Pain. 2015. PMID: 25585270 Free PMC article. Clinical Trial. - A role for Piezo2 in EPAC1-dependent mechanical allodynia.
Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN. Eijkelkamp N, et al. Nat Commun. 2013;4:1682. doi: 10.1038/ncomms2673. Nat Commun. 2013. PMID: 23575686 Free PMC article.
References
- Dearborn G. A case of congenital general pure analgesia. J Nerv Ment Dis. 1932;75:612–615.
- Dyck PJ, et al. Not ‘indifference to pain’ but varieties of hereditary sensory and autonomic neuropathy. Brain. 1983;106:373–390. - PubMed
- Landrieu P, Said G, Allaire C. Dominantly transmitted congenital indifference to pain. Ann Neurol. 1990;27:574–578. - PubMed
- Nagasako EM, Oaklander AL, Dworkin RH. Congenital insensitivity to pain: an update. Pain. 2003;101:213–219. - PubMed
- Klein CJ, Sinnreich M, Dyck PJ. Indifference rather than insensitivity to pain. Ann Neurol. 2003;53:417–418. author reply Ann. Neurol. 53, 418-419 (2003) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases