Polymorphic secreted kinases are key virulence factors in toxoplasmosis - PubMed (original) (raw)
Polymorphic secreted kinases are key virulence factors in toxoplasmosis
J P J Saeij et al. Science. 2006.
Abstract
The majority of known Toxoplasma gondii isolates from Europe and North America belong to three clonal lines that differ dramatically in their virulence, depending on the host. To identify the responsible genes, we mapped virulence in F(1) progeny derived from crosses between type II and type III strains, which we introduced into mice. Five virulence (VIR) loci were thus identified, and for two of these, genetic complementation showed that a predicted protein kinase (ROP18 and ROP16, respectively) is the key molecule. Both are hypervariable rhoptry proteins that are secreted into the host cell upon invasion. These results suggest that secreted kinases unique to the Apicomplexa are crucial in the host-pathogen interaction.
Figures
Fig. 1
Genetic mapping of virulence phenotypes of F1 progeny from II × III crosses. BALB/c and CBA/J mice were infected with 100,000 or 100 tachyzoites from 40 different F1 recombinant progeny from II × III crosses, and mortality was recorded daily for 40 days. As there were no significant differences between the two different mouse strains, results were pooled. Three phenotypes are represented: (i) “high-dose survivability,” log10 survival time (in days) after injection of 100,000 parasites (black line); (ii) “avirulence,” a binary trait defined as no mortality at any dose (red line); (iii) “low-dose survivability,” log10 survival time (in days) after injection of 100 parasites (blue line). Plots indicate the log-likelihood association of phenotypes with markers aligned across the genome. Marker positions (in cM) are given by tick marks. Significance levels determined by 1000 permutations are indicated by horizontal lines [upper lines are significant; lower line is suggestive (P = 0.1)]. Because the significance levels for all three of the phenotypes differed by less than 0.1 LOD unit, only one significance line is drawn for all three. (A) Primary genome scan (see text). (B) Secondary genome scan after the effect of the major virulence peak on chromosome XII, evident in (A) and cosegregating with the SAG3 marker, is neutralized by making it a covariate.
Fig. 2
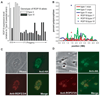
Expression level, polymorphism analysis, and localization of ROP18. (A) Expression level of ROP18 in the parental lines (type II, ME49, and type III, CEP) and 18 F1 progeny. Data are displayed as fold-difference relative to the type III parent. The genotype for each of the F1 progeny at the ROP18 locus is indicated by the bar color (white or black). (B) Variation in the percentage of type I, II, and III SNP polymorphisms across chromosome VIIa. The entire chromosome was divided into 10-kb windows, and the number of SNPs of each type in each window [based on EST assemblies; see (5)] was divided by the number of sites with data from all three strains to compute a polymorphism percentage. The number of each polymorphism type in the ROP18 coding region is also shown. *Accession number AM075204 (28). **Downloaded from (16) (Gene model 20m.03896). (C) Immunofluorescence assay of type III:ROP18II showing HA-specific staining (green) of HA-tagged ROP18 colocalizing with the rhoptry marker ROP2/3/4 (red) in human foreskin fibroblasts 40 hours post inoculation. (D) Immunofluorescence assay of type III:ROP18II showing HA-specific staining of tagged ROP18 in human foreskin fibroblasts 2 hours post inoculation. Punctate staining indicates the location of ROP18-HA that has been secreted into the infected host cell (22). Color scheme same as in (C).
Fig. 3
Effect of expressing the type II allele for ROP18 in a type III strain background (CEP) on virulence in mice. Mice were infected intra-peritoneally with either CEPΔ_hxgprt_ complemented with HXGPRT alone (CEP*) or CEP parasites complemented with the type II allele for ROP18 along with 588 bp of upstream sequence (type III:ROP18II). Infections were verified in all the survivors based on the presence of _Toxoplasma_-specific antibodies, and only seropositive survivors are represented on the graph. Ten mice were used for all strain and dose combinations except for type III:_ROP18II_-100 (eight mice) and type III:_ROP18II_-10 (seven mice).
Fig. 4
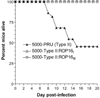
Effect of expressing the type I and III strain allele of ROP16 in the type II Prugniaud strain (PRUΔ_hxgprt_) on virulence in mice. Mice were infected intraperitoneally with 5000 tachyzoites of type II (PRUΔ_hxgprt_) or an engineered version expressing an HA-tagged copy of the ROP16 allele from the type III CEP strain (type II:_ROP16_III) or the type I RH strain (type II:ROP16_I). For PRUΔ_hxgprt, 24 mice were used, 10 for type II:_ROP16_III, and 14 for type II:_ROP16_I. Infections were verified in all the survivors on the basis of the presence of _Toxoplasma_-specific antibodies. Results from three independent experiments were pooled.
Similar articles
- A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii.
Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD. Taylor S, et al. Science. 2006 Dec 15;314(5806):1776-80. doi: 10.1126/science.1133643. Science. 2006. PMID: 17170305 - Association of ROP18 and ROP5 was efficient as a marker of virulence in atypical isolates of Toxoplasma gondii obtained from pigs and goats in Piauí, Brazil.
Rêgo WMF, Costa JGL, Baraviera RCA, Pinto LV, Bessa GL, Lopes REN, Vitor RWA. Rêgo WMF, et al. Vet Parasitol. 2017 Nov 30;247:19-25. doi: 10.1016/j.vetpar.2017.09.015. Epub 2017 Sep 22. Vet Parasitol. 2017. PMID: 29080759 - Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of Toxoplasma gondii.
Behnke MS, Khan A, Lauron EJ, Jimah JR, Wang Q, Tolia NH, Sibley LD. Behnke MS, et al. PLoS Genet. 2015 Aug 20;11(8):e1005434. doi: 10.1371/journal.pgen.1005434. eCollection 2015 Aug. PLoS Genet. 2015. PMID: 26291965 Free PMC article. - Genetic Mapping of Pathogenesis Determinants in Toxoplasma gondii.
Behnke MS, Dubey JP, Sibley LD. Behnke MS, et al. Annu Rev Microbiol. 2016 Sep 8;70:63-81. doi: 10.1146/annurev-micro-091014-104353. Epub 2016 Jun 17. Annu Rev Microbiol. 2016. PMID: 27359216 Review. - Modulation of innate immunity by Toxoplasma gondii virulence effectors.
Hunter CA, Sibley LD. Hunter CA, et al. Nat Rev Microbiol. 2012 Nov;10(11):766-78. doi: 10.1038/nrmicro2858. Nat Rev Microbiol. 2012. PMID: 23070557 Free PMC article. Review.
Cited by
- Toxoplasma WH3 Δrop18 acts as a live attenuated vaccine against acute and chronic toxoplasmosis.
Wang C, Fu S, Yu X, Zhou H, Zhang F, Song L, Zhao J, Yang Y, Du J, Luo Q, Shen J, Yu L. Wang C, et al. NPJ Vaccines. 2024 Oct 23;9(1):197. doi: 10.1038/s41541-024-00996-9. NPJ Vaccines. 2024. PMID: 39443531 Free PMC article. - In Silico Analysis of the ROP29 Protein as a Vaccine Candidate Against Toxoplasma gondii.
Karimipour-Saryazdi A, Ghaffarifar F, Dalimi A, Foroutan M, Horton J, Sadraei J. Karimipour-Saryazdi A, et al. J Parasitol Res. 2024 Jul 26;2024:1918202. doi: 10.1155/2024/1918202. eCollection 2024. J Parasitol Res. 2024. PMID: 39105194 Free PMC article. - Early immune response to Toxoplasma gondii lineage III isolates of different virulence phenotype.
Uzelac A, Klun I, Djurković-Djaković O. Uzelac A, et al. Front Cell Infect Microbiol. 2024 Jun 7;14:1414067. doi: 10.3389/fcimb.2024.1414067. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38912206 Free PMC article. - Knockdown of DJ-1 Exacerbates Neuron Apoptosis Induced by TgCtwh3 through the NF-κB Pathway.
Yang D, Wu M, Zou N, Tang Y, Tao Q, Liu L, Jin M, Yu L, Du J, Luo Q, Shen J, Chu D, Qin K. Yang D, et al. Mol Neurobiol. 2024 Jun 3. doi: 10.1007/s12035-024-04265-7. Online ahead of print. Mol Neurobiol. 2024. PMID: 38831169 - Proteomic approaches for protein kinase substrate identification in Apicomplexa.
Cabral G, Moss WJ, Brown KM. Cabral G, et al. Mol Biochem Parasitol. 2024 Sep;259:111633. doi: 10.1016/j.molbiopara.2024.111633. Epub 2024 May 29. Mol Biochem Parasitol. 2024. PMID: 38821187 Review.
References
- Howe DK, Sibley LD. J. Infect. Dis. 1995;172:1561. - PubMed
- Darde ML, Bouteille B, Pestre-Alexandre M. J. Parasitol. 1992;78:786. - PubMed
- Grigg ME, Bonnefoy S, Hehl AB, Suzuki Y, Boothroyd JC. Science. 2001;294:161. - PubMed
- Lehmann T, Blackston CR, Parmley SF, Remington JS, Dubey JP. J. Parasitol. 2000;86:960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI021423-20/AI/NIAID NIH HHS/United States
- R01 AI036629/AI/NIAID NIH HHS/United States
- AI21423/AI/NIAID NIH HHS/United States
- AI36629/AI/NIAID NIH HHS/United States
- AI30230/AI/NIAID NIH HHS/United States
- R01 AI021423/AI/NIAID NIH HHS/United States
- AI059176/AI/NIAID NIH HHS/United States
- AI41014/AI/NIAID NIH HHS/United States
- 1R01AI045806-01A1/AI/NIAID NIH HHS/United States
- Wellcome Trust/United Kingdom
- AI05093/AI/NIAID NIH HHS/United States
- F32AI60306/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources