Phosphatidylinositol 3-kinase/protein kinase Akt negatively regulates plasminogen activator inhibitor type 1 expression in vascular endothelial cells - PubMed (original) (raw)
Phosphatidylinositol 3-kinase/protein kinase Akt negatively regulates plasminogen activator inhibitor type 1 expression in vascular endothelial cells
Yasushi Mukai et al. Am J Physiol Heart Circ Physiol. 2007 Apr.
Abstract
Plasminogen activator inhibitor type 1 (PAI-1) regulates fibrinolytic activity and mediates vascular atherothrombotic disease. Endothelial cells (ECs) synthesize and secrete PAI-1, but the intracellular signaling pathways that regulate PAI-1 expression are not entirely known. We hypothesize that the phosphatidylinositol 3-kinase (PI3K)/protein kinase Akt pathway, which regulates endothelial function, could modulate PAI-1 expression in ECs. Cultured bovine aortic and human saphenous vein ECs were stimulated with TNF-alpha, ANG II, insulin, or serum, and PAI-1 expression was determined by Northern and Western analyses. Inhibition of PI3K with wortmannin or LY-294002 enhanced PAI-1 expression induced by these extracellular stimuli. Similarly, overexpression of a dominant-negative mutant of PI3K or Akt increased TNF-alpha- and insulin-induced PAI-1 expression. The increase in PAI-1 was due to transcriptional and posttranscriptional mechanisms as PI3K inhibitors increased PAI-1 promoter activity and mRNA stability. The induction of PAI-1 by TNF-alpha and insulin is mediated, in part, by ERK and p38 MAPK. PI3K inhibitors augmented TNF-alpha- and insulin-induced phosphorylation of these MAPKs. Simvastatin, a 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor, which is known to activate PI3K/Akt, blocks TNF-alpha- and insulin-induced PAI-1 expression. Treatment with PI3K inhibitors reversed the inhibitor effects of simvastatin on TNF-alpha- and insulin-induced PAI-1 expression. These findings indicate that the PI3K/Akt pathway acts as a negative regulator of PAI-1 expression in ECs, in part, through the downregulation of MAPK pathways. These results suggest that factors that activate the PI3K/Akt pathway in ECs may have therapeutic benefits for atherothrombotic vascular disease.
Figures
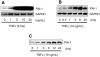
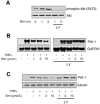
Fig. 1
Induction of plasminogen activator inhibitor type 1 (PAI-1) expression by TNF-α in endothelial cells (ECs). A: Northern blots showing dose-dependent induction of PAI-1 mRNA. B: Northern blots showing time-dependent induction of PAI-1 mRNA. C: Western blots showing time-dependent induction of PAI-1 protein expression.
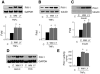
Fig. 2
Inhibition of phosphatidylinositol 3-kinase (PI3K) augments TNF-α-induced PAI-1 expression and activity. A: Northern blots showing effects of wortmannin (WM, 50 nmol/l) and LY-294002 (LY, 3 μmol/l) on TNF-α (10 ng/ml, 6 h)-induced PAI-1 mRNA expression. C, control. Values are means ± SE (n = 4). *P < 0.05 vs. TNF-α alone. B: Western blot showing effects of wortmannin or LY-294002 on TNF-α (10 ng/ml, 24 h)-induced PAI-1 protein expression. Values are means ± SE (n = 5). *P < 0.05 vs. TNF-α alone. C: Western blot showing effects of wortmannin or LY-294002 on insulin (1 μmol/l, 6 h)-induced PAI-1 protein expression. Values are means ± SE (n = 3). *P < 0.05 vs. insulin alone. D: Northern blots showing effects of PI3K inhibitors on ANG II- and FCS-induced PAI-1 mRNA expression. Cells were stimulated with ANG II or FCS in the presence or absence of PI3K inhibitors for 6 h. E: effect of wortmannin or LY-294002 on TNF-α-induced PAI-1 activity. At ∼12 h after TNF-α stimulation, PAI-1 activity was measured in conditioned media by ELISA. Values are means ± SE (n = 4). *P < 0.05 vs. TNF-α alone.
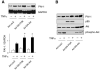
Fig. 3
Effects of PI3K and Akt inhibition of PAI-1 expression. A and B: Northern and Western blots, respectively, showing effects of adenoviruses (Ad) encoding LacZ (Ad-LacZ), a dominant-negative (DN) mutant of PI3K (Ad-DN-PI3K), and a dominant-negative mutant of Akt (Ad-DN-Akt) on TNF-α (10 ng/ml)-induced PAI-1 mRNA expression. Blots were evaluated 6 and 24 h after TNF-α stimulation. Values are means ± SE (n = 4). *P < 0.05 vs. Ad-LacZ with TNF-α.
Fig. 4
Transcriptional and posttranscriptional regulation of PAI-1 expression by PI3K. A: dual-luciferase reporter assay showing effects of wortmannin (50 nmol/l) and LY-294002 (3 μmol/l) on TNF-α (10 ng/ml)-induced PAI-1 promoter activity in ECs. *P < 0.05 vs. TNF-α alone. B: Northern blots with corresponding analysis showing half-life of PAI-1 mRNA in the presence of mRNA synthase inhibitor 5,6-dichlorobenzimidazole riboside (25 μmol/l), which was added after 6 h of stimulation with TNF-α (10 ng/ml). Ctl, control. Values are means ± SE (n = 4). *P < 0.05. **P < 0.01.
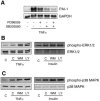
Fig. 5
Role of MAPKs in PAI-1 expression. A: Northern blots showing effects of MAPK inhibitors on PAI-1 expression. Pretreatment with an ERK kinase (MEK) inhibitor (PD-98059, 10 μmol/l) or a p38 MAPK inhibitor (SB-203580, 10 μmol/l) suppressed TNF-α (10 ng/ml)-induced PAI-1 expression. Pretreatment with PD-98059 and SB-203580 almost completely suppressed TNF-α (10 ng/ml)-induced PAI-1 expression. B and C: Western blots showing effect of wortmannin (50 nmol/l) and LY-294002 (3 μmol/l) on phosphorylations of ERK1/2 and p38 MAPK. Cells were stimulated with TNF-α (10 ng/ml) or insulin (1 μmol/l) in the presence or absence of wortmannin (50 nmol/l) or LY-294002 (3 μmol/l) for 12 h.
Fig. 6
Role of PI3K in the inhibitory effect of simvastatin on TNF-α-induced PAI-1 expression. A: Western blots showing activation (phosphorylation at Ser473) of Akt by simvastatin (Sim). B and C: Northern and Western blots, respectively, showing dose-dependent inhibition of TNF-α-induced PAI-1 expression by simvastatin and its reversal with LY-294002 (3 μmol/l).
Fig. 7
Role of PI3K in the inhibitory effect of simvastatin on TNF-α-induced ERK and p38 MAPK phosphorylation. Western blots show dose-dependent inhibition of TNF-α-induced ERK1/2 and p38 MAPK phosphorylation by simvastatin and its reversal with LY-294002 (3 μmol/l).
Similar articles
- Phosphatidylinositol 3-kinase/Akt regulates the balance between plasminogen activator inhibitor-1 and urokinase to promote migration of SKOV-3 ovarian cancer cells.
Whitley BR, Beaulieu LM, Carter JC, Church FC. Whitley BR, et al. Gynecol Oncol. 2007 Feb;104(2):470-9. doi: 10.1016/j.ygyno.2006.08.048. Epub 2006 Oct 30. Gynecol Oncol. 2007. PMID: 17070899 Free PMC article. - TNF-alpha induces Lnk expression through PI3K-dependent signaling pathway in human umbilical vein endothelial cells.
Wan M, Li Y, Xue H, Li Q, Li J. Wan M, et al. J Surg Res. 2006 Nov;136(1):53-7. doi: 10.1016/j.jss.2006.07.004. Epub 2006 Sep 27. J Surg Res. 2006. PMID: 17007883 - The inflammatory cytokine oncostatin M induces PAI-1 in human vascular smooth muscle cells in vitro via PI 3-kinase and ERK1/2-dependent pathways.
Demyanets S, Kaun C, Rychli K, Rega G, Pfaffenberger S, Afonyushkin T, Bochkov VN, Maurer G, Huber K, Wojta J. Demyanets S, et al. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1962-8. doi: 10.1152/ajpheart.01366.2006. Epub 2007 Jun 29. Am J Physiol Heart Circ Physiol. 2007. PMID: 17604327 - Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy.
Kong D, Yamori T. Kong D, et al. Cancer Sci. 2008 Sep;99(9):1734-40. doi: 10.1111/j.1349-7006.2008.00891.x. Epub 2008 Jul 4. Cancer Sci. 2008. PMID: 18616528 Free PMC article. Review. - Plasminogen activator inhibitor-1: the double-edged sword in apoptosis.
Balsara RD, Ploplis VA. Balsara RD, et al. Thromb Haemost. 2008 Dec;100(6):1029-36. Thromb Haemost. 2008. PMID: 19132226 Free PMC article. Review.
Cited by
- JCAD promotes arterial thrombosis through PI3K/Akt modulation: a translational study.
Liberale L, Puspitasari YM, Ministrini S, Akhmedov A, Kraler S, Bonetti NR, Beer G, Vukolic A, Bongiovanni D, Han J, Kirmes K, Bernlochner I, Pelisek J, Beer JH, Jin ZG, Pedicino D, Liuzzo G, Stellos K, Montecucco F, Crea F, Lüscher TF, Camici GG. Liberale L, et al. Eur Heart J. 2023 May 21;44(20):1818-1833. doi: 10.1093/eurheartj/ehac641. Eur Heart J. 2023. PMID: 36469488 Free PMC article. - Association of Ratio of Apolipoprotein B to Apolipoprotein A1 With Survival in Peritoneal Dialysis.
Yu J, Xia X, Huang NY, Qiu YG, Yang X, Mao HP, Chen W, Huang FX. Yu J, et al. Front Nutr. 2022 Mar 25;9:801979. doi: 10.3389/fnut.2022.801979. eCollection 2022. Front Nutr. 2022. PMID: 35399692 Free PMC article. - Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling.
He W, Tan R, Dai C, Li Y, Wang D, Hao S, Kahn M, Liu Y. He W, et al. J Biol Chem. 2010 Aug 6;285(32):24665-75. doi: 10.1074/jbc.M109.091256. Epub 2010 Jun 2. J Biol Chem. 2010. PMID: 20519507 Free PMC article. - Type 2 diabetes mellitus and hypertension: an update.
Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Lastra G, et al. Endocrinol Metab Clin North Am. 2014 Mar;43(1):103-22. doi: 10.1016/j.ecl.2013.09.005. Epub 2013 Dec 12. Endocrinol Metab Clin North Am. 2014. PMID: 24582094 Free PMC article. Review. - Understanding the molecular mechanisms of statin pleiotropic effects.
German CA, Liao JK. German CA, et al. Arch Toxicol. 2023 Jun;97(6):1529-1545. doi: 10.1007/s00204-023-03492-6. Epub 2023 Apr 21. Arch Toxicol. 2023. PMID: 37084080 Free PMC article. Review.
References
- Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:556–562. - PubMed
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. - PubMed
- Eto M, Kozai T, Cosentino F, Joch H, Luscher TF. Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation. 2002;105:1756–1759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS010828/NS/NINDS NIH HHS/United States
- R01 HL080187-01A1/HL/NHLBI NIH HHS/United States
- R01 DK062729-01A1/DK/NIDDK NIH HHS/United States
- R01 HL070274-02/HL/NHLBI NIH HHS/United States
- HL-080187/HL/NHLBI NIH HHS/United States
- P01 NS010828-330036/NS/NINDS NIH HHS/United States
- HL-70274/HL/NHLBI NIH HHS/United States
- R01 HL052233-08/HL/NHLBI NIH HHS/United States
- R01 HL070274-05/HL/NHLBI NIH HHS/United States
- P50 NS010828-300036/NS/NINDS NIH HHS/United States
- R01 DK062729-02/DK/NIDDK NIH HHS/United States
- R01 HL052233-09/HL/NHLBI NIH HHS/United States
- R01 HL070274-04/HL/NHLBI NIH HHS/United States
- R01 HL080187-02/HL/NHLBI NIH HHS/United States
- P50 NS010828/NS/NINDS NIH HHS/United States
- R01 DK062729-03/DK/NIDDK NIH HHS/United States
- R01 HL052233/HL/NHLBI NIH HHS/United States
- R01 DK062729/DK/NIDDK NIH HHS/United States
- R01 HL070274/HL/NHLBI NIH HHS/United States
- R01 HL070274-03/HL/NHLBI NIH HHS/United States
- DK-62729/DK/NIDDK NIH HHS/United States
- R01 HL052233-10/HL/NHLBI NIH HHS/United States
- P50 NS010828-290036/NS/NINDS NIH HHS/United States
- R01 HL080187/HL/NHLBI NIH HHS/United States
- HL-52233/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous