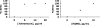Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa - PubMed (original) (raw)
Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa
Lucas R Hoffman et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Opportunistic infections are often polymicrobial. Two of the most important bacterial opportunistic pathogens of humans, Pseudomonas aeruginosa and Staphylococcus aureus, frequently are coisolated from infections of catheters, endotracheal tubes, skin, eyes, and the respiratory tract, including the airways of people with cystic fibrosis (CF). Here, we show that suppression of S. aureus respiration by a P. aeruginosa exoproduct, 4-hydroxy-2-heptylquinoline-N-oxide (HQNO), protects S. aureus during coculture from killing by commonly used aminoglycoside antibiotics such as tobramycin. Furthermore, prolonged growth of S. aureus with either P. aeruginosa or with physiological concentrations of pure HQNO selects for typical S. aureus small-colony variants (SCVs), well known for stable aminoglycoside resistance and persistence in chronic infections, including those found in CF. We detected HQNO in the sputum of CF patients infected with P. aeruginosa, but not in uninfected patients, suggesting that this HQNO-mediated interspecies interaction occurs in CF airways. Thus, in all coinfections with P. aeruginosa, S. aureus may be underappreciated as a pathogen because of the formation of antibiotic-resistant and difficult to detect small-colony variants. Interspecies microbial interactions, analogous to those mediated by HQNO, commonly may alter not only the course of disease and the response to therapy, but also the population structure of bacterial communities that promote the health of host animals, plants, and ecosystems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
HQNO produced by P. aeruginosa simultaneously suppresses the growth of S. aureus and protects it from tobramycin killing. (A) Colonies of P. aeruginosa grown on a lawn of S. aureus on LB agar plates with tobramycin added as indicated. WT, wild-type P. aeruginosa PAO1. The pqsA mutant is defective for HQNO production. The MIC of tobramycin for these P. aeruginosa strains is 1 μg/ml. White arrow, zone of S. aureus growth. S. aureus grows slowly in the zone surrounding the wild-type (WT) P. aeruginosa colony, most evident when the S. aureus lawn is suppressed by tobramycin concentrations >0.3 μg/ml, but similar S. aureus densities are present in the zones under each condition (data not shown). (B) A paper disk (containing 15 μg of pure HQNO) and colonies of P. aeruginosa on a lawn of S. aureus cells on agar media containing 0.6 μg/ml tobramycin. WT, wild-type P. aeruginosa PA14. The pqsL mutant is defective for HQNO production. White arrow, zone of S. aureus growth. (C) Experiment performed as in B, except without tobramycin. (D) S. aureus colonies (of cells from a diluted culture) growing on LB agar near disks containing HQNO or methanol solvent alone. For all P. aeruginosa experiments shown, equivalent results were obtained by using P. aeruginosa strains PAO1 and PA14 and their derived pqsA and pqsL mutants.
Fig. 2.
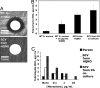
HQNO inhibits S. aureus electron transport, ultimately selecting for SCVs. (A) S. aureus grown for 5 days in static cultures alone, with P. aeruginosa PAO1, or with HQNO as indicated, before plating on selective media to distinguish species and morphotypes. Results shown are on sheep's blood agar. Cells from the culture with HQNO were incubated longer to better display SCVs. Results are representative of three separate experiments; equivalent results were obtained with P. aeruginosa strains PAO1 and PA14 and with S. aureus clinical isolates from five separate CF patients. White arrowheads, normal S. aureus. Black arrows, SCV S. aureus. White arrow, P. aeruginosa. (B) Colonies of cells from S. aureus cultures grown overnight in the presence of the indicated tobramycin and/or HQNO concentrations. Results are representative of two separate experiments. (C) Alamar blue, a redox-sensitive dye, was used to quantify reduction potential (as a measure of electron transport) of the S. aureus strains indicated in the presence and absence of HQNO. Results shown are the average of triplicates ± SD and are representative of three separate experiments. (D) Cells of S. aureus SCVs isolated after HQNO exposure form colonies of wild-type size when growing in close proximity to a disk containing 1.5 μg of menadione and with colony size diminishing with distance from the disk. No such colony changes were observed with disks containing thymidine or hemin. Results are representative of three separate experiments.
Fig. 3.
Synergy between HQNO and tobramycin for S. aureus SCV formation. Quantitative results of the experiment are described in Fig. 2_B_. The relative proportions of SCVs were determined among 100–300 total colony-forming units in overnight cultures of S. aureus grown in HQNO and tobramycin. Growth in the presence of either 10 μg/ml HQNO (Left) or 12 μg/ml tobramycin alone (Right) yielded ≤5% SCVs, whereas in combination, they yielded nearly 100% SCVs, consistent with synergy. Results are representative of two separate experiments, each with at least two technical replicates.
Fig. 4.
HQNO selects for S. aureus SCVs that are resistant to tobramycin. (A) Tobramycin susceptibility of wild-type S. aureus and of an SCV isolated after HQNO exposure. A disk containing 1.5 μg of tobramycin was placed on a S. aureus lawn on LB agar. (B) The MIC of tobramycin for wild-type S. aureus growing in the absence and in the presence of HQNO, and for SCVs derived as indicated. Results shown are averages of technical duplicates ± SD and are representative of three separate experiments. (C) The MIC of tobramycin, with and without menadione, for wild-type S. aureus and SCVs derived as indicated. Menadione restored sensitivity to tobramycin to S. aureus SCVs (but menadione itself was toxic to wild-type S. aureus at 10 μg/ml); results shown are representative of two separate experiments.
Fig. 5.
Model for interspecies interactions between P. aeruginosa and S. aureus. After short-term exposure (hours), S. aureus grown in the presence of P. aeruginosa producing HQNO becomes transiently resistant to aminoglycosides and, possibly, other antibiotics (20, 21). After long-term exposure (days), S. aureus becomes stably resistant because of selection for S. aureus SCVs. Such SCVs can persist intracellularly in nonprofessional phagocytes and are difficult to detect (20). The presence of tobramycin also can select for S. aureus SCVs either independently (20) or synergistically with HQNO (Fig. 3). The production of HQNO and, thus, selection pressure for S. aureus SCVs, could be augmented by increased biofilm formation, either due to reversible induction by aminoglycosides (27) or associated with hyperadherent P. aeruginosa SCVs (28).
Similar articles
- Pseudomonas aeruginosa Alters Staphylococcus aureus Sensitivity to Vancomycin in a Biofilm Model of Cystic Fibrosis Infection.
Orazi G, O'Toole GA. Orazi G, et al. mBio. 2017 Jul 18;8(4):e00873-17. doi: 10.1128/mBio.00873-17. mBio. 2017. PMID: 28720732 Free PMC article. - Staphylococcus aureus sigma B-dependent emergence of small-colony variants and biofilm production following exposure to Pseudomonas aeruginosa 4-hydroxy-2-heptylquinoline-N-oxide.
Mitchell G, Séguin DL, Asselin AE, Déziel E, Cantin AM, Frost EH, Michaud S, Malouin F. Mitchell G, et al. BMC Microbiol. 2010 Jan 30;10:33. doi: 10.1186/1471-2180-10-33. BMC Microbiol. 2010. PMID: 20113519 Free PMC article. - Coculture of Staphylococcus aureus with Pseudomonas aeruginosa Drives S. aureus towards Fermentative Metabolism and Reduced Viability in a Cystic Fibrosis Model.
Filkins LM, Graber JA, Olson DG, Dolben EL, Lynd LR, Bhuju S, O'Toole GA. Filkins LM, et al. J Bacteriol. 2015 Jul;197(14):2252-64. doi: 10.1128/JB.00059-15. Epub 2015 Apr 27. J Bacteriol. 2015. PMID: 25917910 Free PMC article. - Molecular Mechanisms of Staphylococcus and Pseudomonas Interactions in Cystic Fibrosis.
Biswas L, Götz F. Biswas L, et al. Front Cell Infect Microbiol. 2022 Jan 6;11:824042. doi: 10.3389/fcimb.2021.824042. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35071057 Free PMC article. Review. - In vivo and In vitro Interactions between Pseudomonas aeruginosa and Staphylococcus spp.
Hotterbeekx A, Kumar-Singh S, Goossens H, Malhotra-Kumar S. Hotterbeekx A, et al. Front Cell Infect Microbiol. 2017 Apr 3;7:106. doi: 10.3389/fcimb.2017.00106. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28421166 Free PMC article. Review.
Cited by
- Polymicrobial interactions: impact on pathogenesis and human disease.
Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. Peters BM, et al. Clin Microbiol Rev. 2012 Jan;25(1):193-213. doi: 10.1128/CMR.00013-11. Clin Microbiol Rev. 2012. PMID: 22232376 Free PMC article. Review. - Adaptation and Evolution of Pathogens in the Cystic Fibrosis Lung.
Planet PJ. Planet PJ. J Pediatric Infect Dis Soc. 2022 Sep 7;11(Supplement_2):S23-S31. doi: 10.1093/jpids/piac073. J Pediatric Infect Dis Soc. 2022. PMID: 36069898 Free PMC article. Review. - Exit from dormancy in microbial organisms.
Dworkin J, Shah IM. Dworkin J, et al. Nat Rev Microbiol. 2010 Dec;8(12):890-6. doi: 10.1038/nrmicro2453. Epub 2010 Oct 25. Nat Rev Microbiol. 2010. PMID: 20972452 Review. - A spotlight on HCV and SARS-CoV-2 co-infection and brain function.
Shirley K, Loftis JM. Shirley K, et al. Pharmacol Biochem Behav. 2022 Jun;217:173403. doi: 10.1016/j.pbb.2022.173403. Epub 2022 May 10. Pharmacol Biochem Behav. 2022. PMID: 35561837 Free PMC article. No abstract available. - Help, hinder, hide and harm: what can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections?
Limoli DH, Hoffman LR. Limoli DH, et al. Thorax. 2019 Jul;74(7):684-692. doi: 10.1136/thoraxjnl-2018-212616. Epub 2019 Feb 18. Thorax. 2019. PMID: 30777898 Free PMC article. Review.
References
- Hogan DA, Kolter R. Science. 2002;296:2229–2232. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources