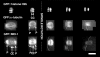Katanin controls mitotic and meiotic spindle length - PubMed (original) (raw)
Katanin controls mitotic and meiotic spindle length
Karen McNally et al. J Cell Biol. 2006.
Abstract
Accurate control of spindle length is a conserved feature of eukaryotic cell division. Lengthening of mitotic spindles contributes to chromosome segregation and cytokinesis during mitosis in animals and fungi. In contrast, spindle shortening may contribute to conservation of egg cytoplasm during female meiosis. Katanin is a microtubule-severing enzyme that is concentrated at mitotic and meiotic spindle poles in animals. We show that inhibition of katanin slows the rate of spindle shortening in nocodazole-treated mammalian fibroblasts and in untreated Caenorhabditis elegans meiotic embryos. Wild-type C. elegans meiotic spindle shortening proceeds through an early katanin-independent phase marked by increasing microtubule density and a second, katanin-dependent phase that occurs after microtubule density stops increasing. In addition, double-mutant analysis indicated that gamma-tubulin-dependent nucleation and microtubule severing may provide redundant mechanisms for increasing microtubule number during the early stages of meiotic spindle assembly.
Figures
Figure 1.
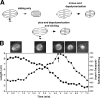
Wild-type meiotic spindles shorten by two distinct, sequential mechanisms. (A) Spindle shortening could occur by one of three mechanisms. In the “sliding only” mechanism, the spindle is shortened by minus end–directed kinesins that slide antiparallel microtubule bundles inward. In this mechanism, the density of spindle microtubules increases as the spindle shortens. In the second mechanism, microtubule minus ends are depolymerized at spindle poles. In the third mechanism, plus-end depolymerization is accompanied by inward sliding. (B) Graph of the length (squares) and fluorescence intensity (circles) of a wild-type meiotic spindle from time-lapse images of GFP∷tubulin fluorescence, shown beginning during MI metaphase. Two phases of spindle shortening are observed. The initial phase is accompanied by an increase in microtubule density, consistent with an inward sliding mechanism, and ends at the time of spindle rotation (indicated by the arrow). The second phase begins 0.5 min after rotation and is accompanied by a decrease in mean microtubule density. Images of the spindle are shown above the corresponding time points, with the cortex outlined for clarity. Note the change in spindle shape during the second phase of shortening.
Figure 2.
The in vitro microtubule-severing activity of C. elegans MEI-1 is completely dependent on MEI-2. Rhodamine-labeled, taxol-stabilized microtubules were immobilized on coverslip surfaces with a mutant kinesin at a final concentration of 0.1 μM tubulin dimer. Microtubules were perfused with solutions of ATP or ADP (1.8 mM) with MEI-1 alone or MEI-1–MEI-2 complexes (1.9 μM). After incubation for the specified time, microtubules were fixed and imaged. In the presence of MEI-1, MEI-2, and ATP, severed microtubules were clearly visible at 5 min, and complete disassembly of microtubules was observed at 10 min. No microtubule severing was observed with MEI-1 alone at concentrations up to 4.7 μM. Bar, 12 μm.
Figure 3.
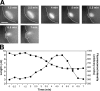
mei-2(ct98) spindles shorten only by the early mechanism. (A) Time-lapse images of an MI spindle in a mei-2(ct98) GFP∷tubulin embryo were captured beginning at metaphase. This spindle shortened from 9.34 to 5.7 μm. It did not rotate until the cortex invaginated around it. Brightness has been adjusted for the later images to increase visibility of the spindle. The cortex has been outlined for clarity. Bar, 6 μm. (B) Graph of spindle length (squares) and fluorescence intensity (circles) beginning 2 min before the start of shortening. MT density initially increased and then decreased as in wild type; however, the spindle shortened only during the initial stage of increasing density.
Figure 4.
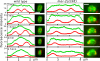
The second phase of spindle shortening is accompanied by katanin-dependent redistribution of microtubules from the poles to the midzone. Time-lapse images of a wild-type embryo and a mei-2(ct98) embryo expressing GFP∷tubulin and mCherry∷histone beginning at rotation were captured with a spinning-disk confocal microscope. Fluorescence intensity was plotted as a function of distance down the pole–pole axis of a single microtubule bundle for each image. Images are shown adjacent to their corresponding plots. Images are in the same orientation as the graphs. In the wild-type spindle, GFP∷tubulin fluorescence intensity (green) of the spindle poles decreased, whereas the intensity of the spindle in between the separating chromosomes increased as the midzone formed. The mei-2(ct98) spindle, which did not shorten during this period, showed no change in the relative intensity of GFP∷tubulin fluorescence at poles versus the midzone, but did exhibit a decrease in microtubule density that progressed uniformly throughout the spindle. Bars, 3.5 μm.
Figure 5.
Katanin localizes at spindle poles at the time of katanin-dependent microtubule redistribution. Sequential time-lapse images of meiotic spindles in three different embryos expressing GFP∷histone H2b, GFP∷α-tubulin, or GFP∷MEI-1. Each column contains images from spindles at an equivalent time point, and all images have been rotated so that the pole–pole axis runs from left to right. Arrows labeled C indicate chromosomes. In the metaphase configurations shown in the leftmost GFP∷histone images, three out of six bivalents are in focus. Each horizontal pair of dots is one bivalent. Arrows labeled P indicate spindle poles. In the left pair of GFP∷MEI-1 images, discrete signals at chromosomes and poles are visible. After spindle rotation and chromosome separation, chromosomes and poles are in almost the same positions. Bar, 6.5 μm.
Figure 6.
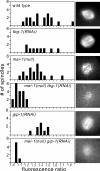
γ-Tubulin and MEI-1 have redundant roles in microtubule assembly during meiotic spindle formation. Images of MI metaphase spindles were captured in embryos expressing GFP∷tubulin, and the mean microtubule density was expressed as the ratio of the mean pixel value of the spindle to the mean pixel value of the surrounding cytoplasm. Histograms indicate the number of spindles with the indicated fluorescence ratio for each genotype. The microtubule densities of spindles in embryos double depleted of both MEI-1 and either of the γ-TuRC subunits, TBG-1 or GIP-1, are markedly lower than those in embryos depleted of any protein alone. Representative images are shown next to each histogram. Brightness and contrast have been adjusted to allow visualization of spindle morphology.
Figure 7.
MEI-1 and γ-tubulin enter the nucleus at the time that meiotic spindle assembly is initiated. (A) Time-lapse images of a maturing oocyte expressing GFP∷α-tubulin show that, before nuclear envelope breakdown (NEBD), tubulin is excluded from the nucleus. During NEBD, tubulin entered the nuclear region. After NEBD, a cloud of increased fluorescence filled the volume of the perforated nucleus. We interpret this cloud of increased fluorescence as a cloud of microtubules filling the volume of the perforated nucleus. This cloud of microtubules then contracted and organized into a group of thick bundles by ovulation. (B) Time-lapse images of a maturing oocyte expressing GFP∷γ-tubulin show that γ-tubulin is concentrated around the nuclear envelope before NEBD and then localizes to a diffuse cloud filling the volume of the perforated nucleus until ovulation. (C) Embryos that have just moved from the spermatheca to the uterus exhibit a vesicle-free zone in differential interference contrast (DIC) that contains the metaphase I spindle. (D) No discrete localization of GFP∷γ-tubulin could be discerned within the vesicle-free zone at this time point, although diffuse cytoplasmic fluorescence was visible and GFP∷γ-tubulin clearly labeled mitotic centrosomes (E) after the completion of meiosis. (F) Images of GFP∷MEI-1 fluorescence showing an oocyte before NEBD (left) and maturing oocyte just after NEBD (right). Before NEBD, GFP∷MEI-1 is distributed throughout the cytoplasm and is excluded from the nucleus. After NEBD occurs, the protein first localizes to a diffuse cloud and then becomes concentrated at poles and chromosomes as the spindle assembles. Bar, 12 μm.
Figure 8.
Detailed model for the action of katanin during CV1 cell mitosis and C. elegans meiosis. (A) We propose that CV1 cell mitotic spindle poles consist of short, overlapping microtubule arrays generated by katanin-mediated microtubule severing at poles and cross-bridged by the combined actions of cytoplasmic dynein and Eg5. Nocodazole-mediated microtubule depolymerization results in a rapid loss of microtubule overlap that is required for dynein and Eg5 to maintain spindle structure. In the absence of cross-bridged overlap, there would be no resistance to inward contraction by a tensile element or to inward force generated by plus-end depolymerization at kinetochores. (B) Mitotic spindles in katanin-inhibited CV1 cells are composed of longer, more contiguous microtubule arrays. More time is thus required for nocodazole to cause a loss of microtubule overlap and, therefore, a loss of resistance to inward forces. (C) During wild-type C. elegans germinal vesicle breakdown, microtubules with a mean length of 4.5 μm assemble because of the combined effects of dynamic instability and katanin-dependent microtubule severing. These microtubules assemble into a 7.7-μm-long metaphase spindle composed of overlapping microtubules. During the first phase of spindle shortening, microtubules slide inward until they completely overlap, generating a 4.5-μm-long spindle that has twice the microtubule density as the metaphase spindle. (D) In mei-2(ct98) oocytes, microtubules assemble at germinal vesicle breakdown with a mean length of 6.2 μm because of the absence of microtubule severing. These microtubules assemble into a 9.6-μm-long metaphase spindle that shortens by inward sliding until the 6.2-μm microtubules completely overlap, resulting in a 6.2-μm-long spindle that does not shorten further. (E) After completion of inward sliding, 4.5-μm-long wild-type meiotic spindles undergo a transition that is marked by spindle rotation, initiation of chromosome separation, and a switch from increasing to decreasing microtubule density. MEI-1 is concentrated at spindle poles at this time, and we suggest that severing of microtubules at the poles allows a redistribution of short microtubules from the poles to the midzone. The redistribution of microtubule density and the further shortening to 2.7 μm that occur in wild type do not occur in mei-2(ct98) embryos because of a lack of microtubule severing at spindle poles.
Comment in
- Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays?
Roll-Mecak A, Vale RD. Roll-Mecak A, et al. J Cell Biol. 2006 Dec 18;175(6):849-51. doi: 10.1083/jcb.200611149. J Cell Biol. 2006. PMID: 17178905 Free PMC article.
Similar articles
- Katanin disrupts the microtubule lattice and increases polymer number in C. elegans meiosis.
Srayko M, O'toole ET, Hyman AA, Müller-Reichert T. Srayko M, et al. Curr Biol. 2006 Oct 10;16(19):1944-9. doi: 10.1016/j.cub.2006.08.029. Curr Biol. 2006. PMID: 17027492 - The spindle assembly function of Caenorhabditis elegans katanin does not require microtubule-severing activity.
McNally KP, McNally FJ. McNally KP, et al. Mol Biol Cell. 2011 May;22(9):1550-60. doi: 10.1091/mbc.E10-12-0951. Epub 2011 Mar 3. Mol Biol Cell. 2011. PMID: 21372175 Free PMC article. - Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays?
Roll-Mecak A, Vale RD. Roll-Mecak A, et al. J Cell Biol. 2006 Dec 18;175(6):849-51. doi: 10.1083/jcb.200611149. J Cell Biol. 2006. PMID: 17178905 Free PMC article. - The elegans of spindle assembly.
Müller-Reichert T, Greenan G, O'Toole E, Srayko M. Müller-Reichert T, et al. Cell Mol Life Sci. 2010 Jul;67(13):2195-213. doi: 10.1007/s00018-010-0324-8. Epub 2010 Mar 26. Cell Mol Life Sci. 2010. PMID: 20339898 Free PMC article. Review. - Spindle positioning during the asymmetric first cell division of Caenorhabditis elegans embryos.
Gönczy P, Grill S, Stelzer EH, Kirkham M, Hyman AA. Gönczy P, et al. Novartis Found Symp. 2001;237:164-75; discussion 176-81. doi: 10.1002/0470846666.ch13. Novartis Found Symp. 2001. PMID: 11444042 Review.
Cited by
- How cells know the size of their organelles.
Chan YH, Marshall WF. Chan YH, et al. Science. 2012 Sep 7;337(6099):1186-9. doi: 10.1126/science.1223539. Science. 2012. PMID: 22955827 Free PMC article. Review. - A novel chromosome segregation mechanism during female meiosis.
McNally KP, Panzica MT, Kim T, Cortes DB, McNally FJ. McNally KP, et al. Mol Biol Cell. 2016 Aug 15;27(16):2576-89. doi: 10.1091/mbc.E16-05-0331. Epub 2016 Jun 22. Mol Biol Cell. 2016. PMID: 27335123 Free PMC article. - Micron-scale geometrical features of microtubules as regulators of microtubule organization.
Mani N, Wijeratne SS, Subramanian R. Mani N, et al. Elife. 2021 Jun 11;10:e63880. doi: 10.7554/eLife.63880. Elife. 2021. PMID: 34114950 Free PMC article. Review. - Specific sensory neurons and insulin-like peptides modulate food type-dependent oogenesis and fertilization in Caenorhabditis elegans.
Mishra S, Dabaja M, Akhlaq A, Pereira B, Marbach K, Rovcanin M, Chandra R, Caballero A, Fernandes de Abreu D, Ch'ng Q, Alcedo J. Mishra S, et al. Elife. 2023 Nov 17;12:e83224. doi: 10.7554/eLife.83224. Elife. 2023. PMID: 37975568 Free PMC article. - Kinesin-1 and cytoplasmic dynein act sequentially to move the meiotic spindle to the oocyte cortex in Caenorhabditis elegans.
Ellefson ML, McNally FJ. Ellefson ML, et al. Mol Biol Cell. 2009 Jun;20(11):2722-30. doi: 10.1091/mbc.e08-12-1253. Epub 2009 Apr 8. Mol Biol Cell. 2009. PMID: 19357192 Free PMC article.
References
- Buster, D., K. McNally, and F.J. McNally. 2002. Katanin inhibition prevents the redistribution of gamma-tubulin at mitosis. J. Cell Sci. 115:1083–1092. - PubMed
- Cassimeris, L., and E.D. Salmon. 1991. Kinetochore microtubules shorten by loss of subunits at the kinetochores of prometaphase chromosomes. J. Cell Sci. 98:151–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources