Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist - PubMed (original) (raw)
Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist
Ye Ye et al. J Virol. 2007 Mar.
Abstract
The recent emergence of several new coronaviruses, including the etiological cause of severe acute respiratory syndrome, has significantly increased the importance of understanding virus-host cell interactions of this virus family. We used mouse hepatitis virus (MHV) A59 as a model to gain insight into how coronaviruses affect the type I alpha/beta interferon (IFN) system. We demonstrate that MHV is resistant to type I IFN. Protein kinase R (PKR) and the alpha subunit of eukaryotic translation initiation factor are not phosphorylated in infected cells. The RNase L activity associated with 2',5'-oligoadenylate synthetase is not activated or is blocked, since cellular RNA is not degraded. These results are consistent with lack of protein translation shutoff early following infection. We used a well-established recombinant vaccinia virus (VV)-based expression system that lacks the viral IFN antagonist E3L to screen viral genes for their ability to rescue the IFN sensitivity of the mutant. The nucleocapsid (N) gene rescued VVDeltaE3L from IFN sensitivity. N gene expression prevents cellular RNA degradation and partially rescues the dramatic translation shutoff characteristic of the VVDeltaE3L virus. However, it does not prevent PKR phosphorylation. The results indicate that the MHV N protein is a type I IFN antagonist that likely plays a role in circumventing the innate immune response.
Figures
FIG. 1.
Effect of IFN treatment on MHV A59 infection. Monolayers of 17Cl1 cells were treated with increasing amounts (1 to 104 units) of type I IFN 24 h before infection. Cells were infected with MHV A59 or VSV at an MOI of 5.
FIG. 2.
Protein synthesis in MHV A59-infected cells. (A) Time course of uninfected (U) and MHV infected (I) 17Cl1 cells. Cells were infected with WT virus at an MOI of 5. Cells were radiolabeled with [35S]methionine-cysteine for 30 min at the indicated times. Intracellular proteins were analyzed by SDS-PAGE and autoradiography. Positions of molecular weight markers in thousands (left) and of viral proteins (right) are indicated. (B) HeLa MHVR cells were mock pretreated or pretreated with IFN prior to infection with MHV, VV, VVΔE3L, or VVΔE3L N at an MOI of 5. Cells were pulse-labeled at 6 hpi for 30 min. Intracellular proteins were analyzed by SDS-PAGE and autoradiography. Proteins in each lane were quantified by densitometry and analyzed using ImageQuant software. Protein expression levels shown below each lane are expressed as the percentage of that measured for uninfected cells.
FIG. 3.
Detection of eIF2α phosphorylation in MHV A59- and VV-infected cells. HeLa MHVR (A) and mouse 17Cl1 (B) cells were infected with viruses as indicated above each lane. Cells were mock or IFN treated prior to infection. At 6 hpi cell lysates were analyzed for eIF2α by Western blotting with antibodies specific for the phosphorylated form of eIF2α and those that recognize both the phosphorylated and unphosphorylated forms (total eIF2α) of the protein. (C) Cell lysates from 17Cl1 cells infected with VVΔE3L or VVΔE3L N viruses were analyzed for eIF2α phosphorylation.
FIG. 4.
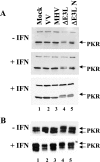
PKR phosphorylation in MHV A59- and VV-infected cells. Immunoblot analysis of PKR phosphorylation in HeLa MHVR (A) and mouse 17Cl1 (B) cells that were pretreated or not with IFN prior to infection with the viruses indicated above each lane is shown. PKR was detected by Western blotting. The slower-migrating phosphorylated PKR is indicated by arrows. Results from two independent experiments are shown for HeLa MHVR-infected cells with IFN treatment in panel A.
FIG. 5.
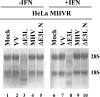
Regulation of 2′-5′ OAS activity in MHV A59- and VV-infected cells. HeLa MHVR cells were infected with the viruses as indicated above each lane. Equal amounts of total RNA from cells at 6 hpi were analyzed by formaldehyde agarose gel electrophoresis and visualized by ethidium bromide staining. The positions of 18S and 28S rRNAs are indicated.
FIG. 6.
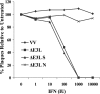
IFN sensitivity of VV recombinant viruses in RK-13 cells. Monolayers of RK-13 cells were treated with increasing amounts (0 to 104 units) of type I IFN 24 h before infection. Plaque counts are expressed as a percentage of the untreated parallel control value for each virus. The data represent the averages of duplicate infections.
FIG. 7.
N protein is a type I IFN antagonist. (A) Monolayers of 17Cl1 cells were treated with increasing amounts (1 to 104 units) of type I IFN 24 h before infection. Cells were infected with the parental infectious clone-derived MHV A59 or ΔI virus at an MOI of 5. Plaques were stained with crystal violet at 48 hpi. (B) HeLa MHVR cells were mock or IFN treated prior to infection with MHV ΔI and WT MHV. At 6 hpi cell lysates were analyzed for eIF2α by Western blotting with antibodies specific for the phosphorylated form of eIF2α (two upper panels) and those that recognize both the phosphorylated and unphosphorylated forms of the protein for total eIF2α (lower two panels). The lanes shown were analyzed in parallel with results shown in Fig. 3A. (C) HeLa MHVR cells were infected with the viruses as indicated above each lane. Equal amounts of total RNA from cells at 6 hpi were analyzed by formaldehyde agarose gel electrophoresis and visualized by ethidium bromide staining. The positions of 18S and 28S rRNAs are indicated. (D) HeLa MHVR cells were transfected with either empty pCAGGS vector (C) or vector containing the WT N and ΔI N genes. Transfected cells were infected with VVΔE3L at 48 hpi and labeled 6 h later. Intracellular proteins were analyzed by SDS-PAGE and autoradiography. Proteins in each lane were quantified by densitometry. Measurements from three independent experiments were averaged. Error bars represent the standard deviations of the individual measurements.
FIG. 8.
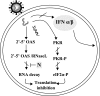
MHV A59 interference with type I IFN pathways. MHV A59 interferes with the downstream 2′-5′ OAS/RNase L and PKR pathways following treatment of cells with type I IFN. The N protein blocks the RNase L activity associated with 2′-5′ OAS. Recent results indicate that MHV A59 does not induce IFN-β expression (71).
Similar articles
- Roles of vaccinia virus genes E3L and K3L and host genes PKR and RNase L during intratracheal infection of C57BL/6 mice.
Rice AD, Turner PC, Embury JE, Moldawer LL, Baker HV, Moyer RW. Rice AD, et al. J Virol. 2011 Jan;85(1):550-67. doi: 10.1128/JVI.00254-10. Epub 2010 Oct 13. J Virol. 2011. PMID: 20943971 Free PMC article. - Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A.
He Y, Tan SL, Tareen SU, Vijaysri S, Langland JO, Jacobs BL, Katze MG. He Y, et al. J Virol. 2001 Jun;75(11):5090-8. doi: 10.1128/JVI.75.11.5090-5098.2001. J Virol. 2001. PMID: 11333890 Free PMC article. - Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus.
Child SJ, Jarrahian S, Harper VM, Geballe AP. Child SJ, et al. J Virol. 2002 May;76(10):4912-8. doi: 10.1128/jvi.76.10.4912-4918.2002. J Virol. 2002. PMID: 11967308 Free PMC article. - Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene.
Beattie E, Denzler KL, Tartaglia J, Perkus ME, Paoletti E, Jacobs BL. Beattie E, et al. J Virol. 1995 Jan;69(1):499-505. doi: 10.1128/JVI.69.1.499-505.1995. J Virol. 1995. PMID: 7527085 Free PMC article. - Murine coronavirus neuropathogenesis: determinants of virulence.
Cowley TJ, Weiss SR. Cowley TJ, et al. J Neurovirol. 2010 Nov;16(6):427-34. doi: 10.3109/13550284.2010.529238. Epub 2010 Nov 12. J Neurovirol. 2010. PMID: 21073281 Free PMC article. Review.
Cited by
- Assembly and Entry of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2): Evaluation Using Virus-Like Particles.
Kumar B, Hawkins GM, Kicmal T, Qing E, Timm E, Gallagher T. Kumar B, et al. Cells. 2021 Apr 9;10(4):853. doi: 10.3390/cells10040853. Cells. 2021. PMID: 33918600 Free PMC article. - Regulation of Stress Responses and Translational Control by Coronavirus.
Fung TS, Liao Y, Liu DX. Fung TS, et al. Viruses. 2016 Jul 4;8(7):184. doi: 10.3390/v8070184. Viruses. 2016. PMID: 27384577 Free PMC article. Review. - Accessory protein 5a is a major antagonist of the antiviral action of interferon against murine coronavirus.
Koetzner CA, Kuo L, Goebel SJ, Dean AB, Parker MM, Masters PS. Koetzner CA, et al. J Virol. 2010 Aug;84(16):8262-74. doi: 10.1128/JVI.00385-10. Epub 2010 Jun 2. J Virol. 2010. PMID: 20519394 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI052347/AI/NIAID NIH HHS/United States
- R01 AI052347/AI/NIAID NIH HHS/United States
- AI 53704/AI/NIAID NIH HHS/United States
- AI 52347/AI/NIAID NIH HHS/United States
- R01 AI053704/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources