COMMD1 promotes the ubiquitination of NF-kappaB subunits through a cullin-containing ubiquitin ligase - PubMed (original) (raw)
COMMD1 promotes the ubiquitination of NF-kappaB subunits through a cullin-containing ubiquitin ligase
Gabriel N Maine et al. EMBO J. 2007.
Abstract
NF-kappaB is a pleiotropic transcription factor involved in multiple processes, including inflammation and oncogenesis. We have previously reported that COMMD1 represses kappaB-dependent transcription by negatively regulating NF-kappaB-chromatin interactions. Recently, ubiquitination of NF-kappaB subunits has been similarly implicated in the control of NF-kappaB recruitment to chromatin. We report here that COMMD1 accelerates the ubiquitination and degradation of NF-kappaB subunits through its interaction with a multimeric ubiquitin ligase containing Elongins B and C, Cul2 and SOCS1 (ECS(SOCS1)). COMMD1-deficient cells demonstrate stabilization of RelA, greater nuclear accumulation of RelA after TNF stimulation, de-repression of several kappaB-responsive genes, and enhanced NF-kappaB-mediated cellular responses. COMMD1 binds to Cul2 in a stimulus-dependent manner and serves to facilitate substrate binding to the ligase by stabilizing the interaction between SOCS1 and RelA. Our data uncover that ubiquitination and degradation of NF-kappaB subunits by this COMMD1-containing ubiquitin ligase is a novel and critical mechanism of regulation of NF-kappaB-mediated transcription.
Figures
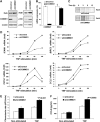
Figure 1
COMMD1 promotes the ubiquitination of NF-κB subunits. (A) Ubiquitination of RelA. HEK 293 cells were transfected with COMMD1 or a vector control, along with GST-RelA. Cells were treated with Lactacystin (10 μM) for 3 h before lysis with Triton X-100 buffer. RelA was precipitated (PD) using glutathione (GSH) beads and the recovered material was immunoblotted for ubiquitin (Ubi). (B) Ubiquitination of endogenous RelA. Cells were transfected with COMMD1 (or vector control) as in (A), and endogenous RelA was immunoprecipitated from cell lysates prepared with Triton X-100 buffer. The recovered material was immunoblotted for ubiquitin. (C) Effects of overexpression and underexpression of COMMD1 on RelA ubiquitination. HEK 293 cells were transfected with His6-ubiquitin (His6-Ubi) or a vector control, along with HA-RelA. These cells were co-transfected with a vector control or COMMD1 (left panel), or with control or COMMD1-specific siRNA (right panel). Ubiquitinated material was precipitated from cell lysates prepared with Triton X-100 buffer, using Ni-NTA agarose beads. The recovered material was immunoblotted for RelA (HA) and ubiquitin. (D) Effects on other NF-κB subunits. HEK 293 cells were transfected as in (C), except that HA-RelB (left panel) or HA-p52 (right panel) was used.
Figure 2
COMMD1 deficiency stabilizes RelA and de-represses endogenous κB-dependent transcription and cellular events. (A) COMMD1 deficiency results in higher basal levels of RelA. U2OS cells were stably infected with lentiviruses targeting a control gene (shControl) or COMMD1 (shCOMMD1). Once stable polyclonal cell lines were established, cell lysates were prepared with Triton X-100 buffer for immunoblotting as indicated. (B) COMMD1 deficiency does not affect mRNA levels of RELA. In the same cells, mRNA was extracted and the levels of COMMD1 and RELA expression were determined by qRT–PCR and expressed as % compared to the control line. (C) The half-life of RelA is prolonged in COMMD1-deficient cells. Cells were metabolically labeled with 35S-labeled methionine and cysteine and cell lysates were prepared at increasing time intervals as indicated. After immunoprecipitation of RelA, the recovered material was resolved by SDS–PAGE and subjected to autoradiography. (D) COMMD1 deficiency results in enhanced transcription of several κB-responsive genes. COMMD1-deficient and control cell lines were stimulated with a 10 min pulse of TNF, rinsed in PBS, and placed in normal media. Extraction of mRNA was performed for qRT–PCR of the indicated gene transcripts. (E) COMMD1-deficient cells produce greater amounts of chemotactic factors. Conditioned media from untreated or TNF-treated cells (12 h) were utilized. The ability of the media to induce chemotaxis was determined by monitoring the migration of fluorescently labeled peripheral mononuclear cells across a membrane barrier (and expressed as a rate of fluorescence change over time). (F) COMMD1-deficient cells secrete greater amounts of CCL2. Levels of CCL2 in the conditioned media (prepared similarly as in (E)) were determined by ELISA.
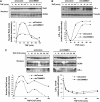
Figure 3
COMMD1 deficiency alters the nuclear accumulation of RelA. (A) COMMD1 deficiency results in greater accumulation of nuclear RelA after TNF treatment. U2OS COMMD1-deficient and control cell lines were stimulated with a 10 min pulse of TNF. At the indicated time point, cells were harvested and nuclear extracts were prepared for Western blot analysis of RelA in the nuclear fraction (and GCN5 as a loading control). Densitometry analysis is also presented. (B) Greater nuclear accumulation of RelA is not due to alterations in IκB-α degradation or resynthesis. U2OS cells were treated in an identical manner as in the experiment performed in (A) and IκB-α degradation was evaluated by immunoblotting and densitometry. (C) COMMD1 deficiency promotes greater nuclear accumulation of RelA independent of IκB-α levels. Similar to (A), U2OS cells were stimulated with a 10 min pulse of TNF, and medium containing CHX (30 μM) was then added. Nuclear extracts were prepared at the indicated time intervals and immunoblotted for RelA. In addition, IκB-α levels were evaluated utilizing the cytosolic fractions (bottom panel). Representative data and densitometry analysis are presented.
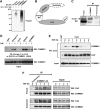
Figure 4
COMMD1 interacts with the Cullin-containing ECSSOCS1 complex. (A) COMMD1 immunoprecipitates possess endogenous E3 ubiquitin ligase activity. An in vitro ubiquitination reaction containing recombinant E1, E2, and ubiquitin was supplemented with immunoprecipitates prepared with COMMD1 antibody or preimmune serum control. The presence of E3 activity was determined by the formation of polyubiquitin chains, as detected by immunoblotting. (B) Schematic representation of the ECSSOCS1 complex. SOCS1 is the substrate binding subunit of the complex. An Elongin B and C complex serves as an adaptor linking SOCS1 to Cul2, which in turn interacts with the RING finger protein Rbx1. (C) Interaction between endogenous COMMD1 and SOCS1. NIH-SR cells were lysed with Triton X-100 buffer and COMMD1 antibodies or a control preimmune serum was used for immunoprecipitation. The recovered material was immunoblotted for SOCS1. (D) COMMD1 interacts with several components of ECSSOCS1. HEK 293 cells were transfected with plasmids expressing Elongin C, Cul2, SOCS1, or COMMD1 in fusion with GST, along with COMMD1-Flag in each case. TNF stimulation consisted of 30 min. After lysis with RIPA buffer, ECS components (or COMMD1-GST) were precipitated with GSH beads and the recovered material was immunoblotted for COMMD1 (Flag). (E) COMMD1–Cul2 interactions are inducible upon TNF stimulation. Similar to the experiment shown in (D), cells were transfected to express GST-Cul2 and COMMD1-Flag. Cells were stimulated with TNF for 10 min and then placed in fresh media. The cells were lysed in RIPA buffer at the indicated time points and Cul2 was precipitated using GSH beads. The recovered material was immunoblotted for COMMD1 (Flag). (F) Endogenous COMMD1 and Cul2 interactions. After TNF stimulation, either as a 10 min pulse or a sustained exposure, endogenous COMMD1 was immunoprecipitated at the indicated time points or the recovered material was immunoblotted for endogenous Cul2.
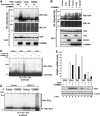
Figure 5
The ECSSOCS1 complex is required for COMMD1-mediated ubiquitination of RelA. (A) COMMD1-mediated ubiquitination of RelA depends on the endogenous ECSSOCS1 complex. HEK 293 cells were transfected with His6-Ubi and RelA as before (Figure 1C), as well as siRNA against Cul2, SOCS1, or a control target. Two days later, mRNA expression levels of Cul2 and SOCS1 were determined by qRT–PCR. In addition, cell lysates were prepared with an 8 M urea buffer and ubiquitinated proteins were precipitated with Ni-NTA beads. The recovered material was immunoblotted for RelA (HA) and ubiquitin. (B) COMMD1 and ECS components have additive effects on RelA ubiquitination. As before, cells were transfected with His6-Ubi and RelA, along with SOCS1, Cul2, and COMMD1, as indicated. Lysates prepared with an 8 M urea buffer were utilized for ubiquitin precipitation; the recovered material was immunoblotted for RelA and ubiquitin. (C, D) In vitro ubiquitination of RelA by COMMD1 immunoprecipitates. An in vitro ubiquitination reaction containing recombinant E1, E2, ubiquitin, and GST-RelA as substrates was provided with immunoprecipitates prepared with COMMD1 antibody or preimmune serum as a source for E3 activity. In (C), the immunoprecipitates were prepared from cells expressing Cul1, Cul2, Cul5, or a vector control. In (D), the immunoprecipitates were prepared from cells expressing ECSSOCS1 subunits (Elongin C, Cul2, SOCS1, and Rbx1) or a vector control. (E) COMMD1 and ECSSOCS1 cooperatively inhibit RelA-mediated ICAM1 expression and promote RelA degradation. HEK 293 cells were transfected with RelA, along with Elongin C (EloC), Cul2, SOCS1, or COMMD1 as indicated. Two days later, ICAM1 mRNA abundance was determined by qRT–PCR and expressed as fold over the vector control sample. In addition, RelA (HA) and β-actin protein levels were concurrently determined by Western blot.
Figure 6
COMMD1 interacts with both Cul2 and SOCS1 and facilitates SOCS1–RelA binding. (A) COMMD1 interacts independently with both SOCS1 and Cul2. Full-length and specific deletion mutants of Cul2 and SOCS1 were expressed in fusion with GST, along with COMMD1-Flag. Cul2 and SOCS1 were precipitated from cell lysates (RIPA buffer) and the recovered material was immunoblotted as indicated. (B) COMMD1 facilitates SOCS1–RelA interactions. HEK 293 cells were transfected with GST-RelA, SOCS1, and increasing amounts of COMMD1 as indicated. Two days after transfection, the cells were lysed in RIPA buffer. RelA was precipitated from the lysate and the recovered material was immunoblotted for SOCS1 (Flag). (C) SOCS1 binds to the amino-terminus of the RHD of RelA. Cells were transfected as in (B), this time utilizing GST-RelA full-length and deletion constructs encompassing the amino acids indicated in the figure. Two days after transfection, cell lysates were prepared with RIPA buffer and RelA was precipitated. The recovered material was immunoblotted for SOCS1 (HA) or RelA (GST). (D) RelA binds to the entire amino-terminus of SOCS1. HEK 293 cells were transfected to express GST-SOCS1 or the deletion mutants indicated, as well as RelA 1–180. Two days after transfection, the cells were lysed in RIPA buffer. SOCS1 was precipitated from the lysate and the recovered material was immunoblotted for RelA 1–180 (HA) or SOCS1 (GST). (E) Schematic representation of COMMD1 interactions with the ECSSOCS1 complex. COMMD1 interacts independently with both SOCS1, at the level of its SH2 domain, and Cul2, at the level of its conserved carboxy-terminus. The interaction between COMMD1 and Cul2 is inducible upon TNF stimulation, and COMMD1 in turn facilitates the binding of RelA to SOCS1.
Similar articles
- CSN-associated USP48 confers stability to nuclear NF-κB/RelA by trimming K48-linked Ub-chains.
Schweitzer K, Naumann M. Schweitzer K, et al. Biochim Biophys Acta. 2015 Feb;1853(2):453-69. doi: 10.1016/j.bbamcr.2014.11.028. Epub 2014 Dec 5. Biochim Biophys Acta. 2015. PMID: 25486460 - Chromatin-associated cullin-RING E3 ubiquitin ligases: keeping transcriptionally active NF-κB in check.
Gong M, Luo J, Liang Q, Liu Y, Zheng Y, Yang XD. Gong M, et al. Front Immunol. 2025 Apr 16;16:1584999. doi: 10.3389/fimmu.2025.1584999. eCollection 2025. Front Immunol. 2025. PMID: 40308609 Free PMC article. Review. - GCN5 is a required cofactor for a ubiquitin ligase that targets NF-kappaB/RelA.
Mao X, Gluck N, Li D, Maine GN, Li H, Zaidi IW, Repaka A, Mayo MW, Burstein E. Mao X, et al. Genes Dev. 2009 Apr 1;23(7):849-61. doi: 10.1101/gad.1748409. Genes Dev. 2009. PMID: 19339690 Free PMC article. - Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA.
Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, Rottapel R, Yamaoka S, Lu KP. Ryo A, et al. Mol Cell. 2003 Dec;12(6):1413-26. doi: 10.1016/s1097-2765(03)00490-8. Mol Cell. 2003. PMID: 14690596 - COMMD proteins and the control of the NF kappa B pathway.
Maine GN, Burstein E. Maine GN, et al. Cell Cycle. 2007 Mar 15;6(6):672-6. doi: 10.4161/cc.6.6.3989. Epub 2007 Mar 7. Cell Cycle. 2007. PMID: 17361106 Free PMC article. Review.
Cited by
- Clusterin and COMMD1 independently regulate degradation of the mammalian copper ATPases ATP7A and ATP7B.
Materia S, Cater MA, Klomp LW, Mercer JF, La Fontaine S. Materia S, et al. J Biol Chem. 2012 Jan 20;287(4):2485-99. doi: 10.1074/jbc.M111.302216. Epub 2011 Nov 30. J Biol Chem. 2012. PMID: 22130675 Free PMC article. - Hepatitis B virus X protein promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells by regulating SOCS1.
Kang I, Kim JA, Kim J, Lee JH, Kim MJ, Ahn JK. Kang I, et al. BMB Rep. 2022 May;55(5):220-225. doi: 10.5483/BMBRep.2022.55.5.157. BMB Rep. 2022. PMID: 35168698 Free PMC article. - Signaling network of dendritic cells in response to pathogens: a community-input supported knowledgebase.
Patil S, Pincas H, Seto J, Nudelman G, Nudelman I, Sealfon SC. Patil S, et al. BMC Syst Biol. 2010 Oct 7;4:137. doi: 10.1186/1752-0509-4-137. BMC Syst Biol. 2010. PMID: 20929569 Free PMC article. - Impaired COMMD10-Mediated Regulation of Ly6Chi Monocyte-Driven Inflammation Disrupts Gut Barrier Function.
Mouhadeb O, Ben Shlomo S, Cohen K, Farkash I, Gruber S, Maharshak N, Halpern Z, Burstein E, Gluck N, Varol C. Mouhadeb O, et al. Front Immunol. 2018 Nov 14;9:2623. doi: 10.3389/fimmu.2018.02623. eCollection 2018. Front Immunol. 2018. PMID: 30487795 Free PMC article. - WIP1 phosphatase is a negative regulator of NF-kappaB signalling.
Chew J, Biswas S, Shreeram S, Humaidi M, Wong ET, Dhillion MK, Teo H, Hazra A, Fang CC, López-Collazo E, Bulavin DV, Tergaonkar V. Chew J, et al. Nat Cell Biol. 2009 May;11(5):659-66. doi: 10.1038/ncb1873. Epub 2009 Apr 19. Nat Cell Biol. 2009. PMID: 19377466
References
- Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, Maine GN, Wilkinson JC, Mayo MW, Duckett CS (2005) COMMD proteins: a novel family of structural and functional homologs of MURR1. J Biol Chem 280: 22222–22232 - PubMed
- Chen L, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293: 1653–1657 - PubMed
- Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T (1995) Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin–proteasome pathway. Genes Dev 9: 1586–1597 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases