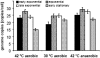Regulated polyploidy in halophilic archaea - PubMed (original) (raw)
Regulated polyploidy in halophilic archaea
Sebastian Breuert et al. PLoS One. 2006.
Abstract
Polyploidy is common in higher eukaryotes, especially in plants, but it is generally assumed that most prokaryotes contain a single copy of a circular chromosome and are therefore monoploid. We have used two independent methods to determine the genome copy number in halophilic archaea, 1) cell lysis in agarose blocks and Southern blot analysis, and 2) Real-Time quantitative PCR. Fast growing H. salinarum cells contain on average about 25 copies of the chromosome in exponential phase, and their ploidy is downregulated to 15 copies in early stationary phase. The chromosome copy number is identical in cultures with a twofold lower growth rate, in contrast to the results reported for several other prokaryotic species. Of three additional replicons of H. salinarum, two have a low copy number that is not growth-phase regulated, while one replicon even shows a higher degree of growth phase-dependent regulation than the main replicon. The genome copy number of H. volcanii is similarly high during exponential phase (on average 18 copies/cell), and it is also downregulated (to 10 copies) as the cells enter stationary phase. The variation of genome copy numbers in the population was addressed by fluorescence microscopy and by FACS analysis. These methods allowed us to verify the growth phase-dependent regulation of ploidy in H. salinarum, and they revealed that there is a wide variation in genome copy numbers in individual cells that is much larger in exponential than in stationary phase. Our results indicate that polyploidy might be more widespread in archaea (or even prokaryotes in general) than previously assumed. Moreover, the presence of so many genome copies in a prokaryote raises questions about the evolutionary significance of this strategy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
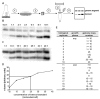
Figure 1
A. Overview of the method. A culture of known cell density is embedded in low melting point agarose (step 1), agarose blocks with a defined number of cells are prepared, the cells are lysed and protein is digested (step 2). The blocks are melted and a restriction enzyme (step 3) as well as an internal standard (step 4) are added. After overnight digestion, DNA fragments are size fractionated by electrophoresis and a Southern blot is performed (step 5). A 1 kbp genomic fragment near the replication origin and the 0.9 kbp internal standard are both visualized with a single probe. Multiple aliquots containing different standard concentration are used for quantitation. B. Quantitation of the genome copy number of exponential cells. After gel electrophoresis and southern blotting, a genomic fragment (upper band) and different concentrations of an internal standard (lower band) were visualized with the same probe (step 5 in A). C. Quantitation of the genome copy number of stationary phase cells. After gel electrophoresis and Southern blotting, a genomic fragment (upper band) and different concentrations of an internal standard (lower band) were visualized with the same probe (step 5 in A). D. An example of a standard curve generated after quantitation of the bands shown in B. and C. The horizontal and vertical lines show the usage of the standard curve to determine the genome copy number in the biological replicate No. 9 (see E.). E. Summary of the results of the independent cultures that were used to determine the genome copy number with the “agarose block method”. In the first five experiments, the standard curve ranged from 0.5 molecules/cell to 10 molecules/cell (as in B.). The signal of exponential phase cells was much higher than the highest signal of the standard curve and could not be quantitated. Therefore the standard curve was chosen to range from 1 molecules/cell to 40 molecules/cell (as in C.) in subsequent experiments.
Figure 2
A. Overview of the method. In short, a defined number of cells was harvested and lysed (steps 1 and 2). Serial dilutions of the cell lysate (step 3) were used as templates in quantitative Real Time PCR assays (step 4). Quantitation was performed by comparison with an external and an internal standard curve (Materials and Methods). B. Selected real time PCR results. The fluorescence intensity curves from four standard dilutions (solid lines) and three sample dilutions (broken lines) are shown. In both cases serial tenfold dilutions of the templates were used. Note the identical slope of all curves and the equidistance of the curves of a dilution series, which is very close to the theoretical offset of 3.32 cycles per tenfold dilution. In addition to the selected reactions shown here, each experiment included more standards and more sample dilutions (filling the gap between tenfold dilutions) as well as a sample dilutions including a dilutions series of the standard added as internal control of PCR efficiency. C. A standard curve including nine standard concentrations distributed over three orders of magnitude. D. Average genome copy number values of three independent cultures and their standard deviation. E. Growth phase-dependent regulation of ploidy of H. salinarum. E is a graphical representation of the results tabulated in D.
Figure 3
H. salinarum was grown by aerobic respiration at 42oC and 30 oC and by arginine fermentation at 42oC. The doubling times were 4 hours, 8 hours and 8 hours, respectively. For each condition three independent cultures were used. Aliquots representing early exponential phase (2–3×108 cells/ml), mid-exponential phase (4–5×108 cells/ml), late exponential phase (8–9×108 cells/ml) and early stationary phase (1–2×109 cells/ml) were used to determine the genome copy number using the Real Time PCR method. Average values of the three biological replicates and their standard deviation are shown.
Figure 4
Three independent cultures were used to determine the copy numbers of the chromosome and three additional replicons, i.e. pHS1 to pHS3, using the Real Time PCR method. One of the growth curves, the average replicon copy number per cell and the standard deviations are shown.
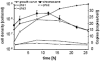
Figure 5
Determination of genome copy number using the agarose block method. Different aliquots from one culture were used to inoculate several new cultures that were grown overnight. Throughouht the next day aliquots were withdrawn, the cell density was determined with a counting chamber and the ploidy with the agarose block method. For each aliquot, the cell density was plotted against the genome copy number, and a trend line was calculated. B. Determination of the genome copy number using the Real Time PCR method. Three independent cultures were used to determine the genome copy number. A selected growth curve, the average ploidy values and their standard deviation are shown.
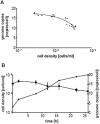
Figure 6
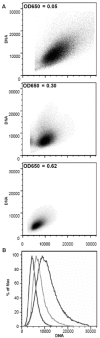
A. The forward light scatter as a measure of cell size is plotted against the fluorescence of the DNA-specific dye acridine orange as a measure of the DNA content. Aliquots representing early and middle exponential phase and early stationary phase (top to bottom) were analyzed, and the optical densities are indicated. B. The fluorescence as a measure of DNA content is plotted against the fraction of the population exhibiting a specific fluorescence. The three curves from left to right represent the three aliquots shown in A from top to bottom.
Similar articles
- Transcriptome changes and cAMP oscillations in an archaeal cell cycle.
Baumann A, Lange C, Soppa J. Baumann A, et al. BMC Cell Biol. 2007 Jun 11;8:21. doi: 10.1186/1471-2121-8-21. BMC Cell Biol. 2007. PMID: 17562013 Free PMC article. - Genome-wide analysis of growth phase-dependent translational and transcriptional regulation in halophilic archaea.
Lange C, Zaigler A, Hammelmann M, Twellmeyer J, Raddatz G, Schuster SC, Oesterhelt D, Soppa J. Lange C, et al. BMC Genomics. 2007 Nov 12;8:415. doi: 10.1186/1471-2164-8-415. BMC Genomics. 2007. PMID: 17997854 Free PMC article. - Variations in the multiple tbp genes in different Halobacterium salinarum strains and their expression during growth.
Teufel K, Bleiholder A, Griesbach T, Pfeifer F. Teufel K, et al. Arch Microbiol. 2008 Sep;190(3):309-18. doi: 10.1007/s00203-008-0383-5. Epub 2008 May 28. Arch Microbiol. 2008. PMID: 18506423 - Ploidy and gene conversion in Archaea.
Soppa J. Soppa J. Biochem Soc Trans. 2011 Jan;39(1):150-4. doi: 10.1042/BST0390150. Biochem Soc Trans. 2011. PMID: 21265763 Review. - Polyploidy in halophilic archaea: regulation, evolutionary advantages, and gene conversion.
Ludt K, Soppa J. Ludt K, et al. Biochem Soc Trans. 2019 Jun 28;47(3):933-944. doi: 10.1042/BST20190256. Epub 2019 Jun 12. Biochem Soc Trans. 2019. PMID: 31189733 Review.
Cited by
- Identification, Characterization, and Application of the Replicon Region of the Halophilic Temperate Sphaerolipovirus SNJ1.
Wang Y, Sima L, Lv J, Huang S, Liu Y, Wang J, Krupovic M, Chen X. Wang Y, et al. J Bacteriol. 2016 Jun 27;198(14):1952-1964. doi: 10.1128/JB.00131-16. Print 2016 Jul 15. J Bacteriol. 2016. PMID: 27137505 Free PMC article. - The chromosome copy number of the hyperthermophilic archaeon Thermococcus kodakarensis KOD1.
Spaans SK, van der Oost J, Kengen SW. Spaans SK, et al. Extremophiles. 2015 Jul;19(4):741-50. doi: 10.1007/s00792-015-0750-5. Epub 2015 May 8. Extremophiles. 2015. PMID: 25952670 Free PMC article. - Intracellular dynamics of archaeal FANCM homologue Hef in response to halted DNA replication.
Lestini R, Laptenok SP, Kühn J, Hink MA, Schanne-Klein MC, Liebl U, Myllykallio H. Lestini R, et al. Nucleic Acids Res. 2013 Dec;41(22):10358-70. doi: 10.1093/nar/gkt816. Epub 2013 Sep 17. Nucleic Acids Res. 2013. PMID: 24049073 Free PMC article. - Multiple replication origins with diverse control mechanisms in Haloarcula hispanica.
Wu Z, Liu J, Yang H, Liu H, Xiang H. Wu Z, et al. Nucleic Acids Res. 2014 Feb;42(4):2282-94. doi: 10.1093/nar/gkt1214. Epub 2013 Nov 22. Nucleic Acids Res. 2014. PMID: 24271389 Free PMC article. - Creating Polyploid Escherichia Coli and Its Application in Efficient L-Threonine Production.
Wang S, Chen X, Jin X, Gu F, Jiang W, Qi Q, Liang Q. Wang S, et al. Adv Sci (Weinh). 2023 Nov;10(31):e2302417. doi: 10.1002/advs.202302417. Epub 2023 Sep 25. Adv Sci (Weinh). 2023. PMID: 37749873 Free PMC article.
References
- Wolfe KH. Yesterday's polyploids and the mystery of diploidization. Nat Rev Genet. 2001;2(5):333–341. - PubMed
- Comai L. The advantages and disadvantages of being polyploid. Nat Rev Genet. 2005;6(11):836–846. - PubMed
- Wendel JF. Genome evolution in polyploids. Plant Mol Biol. 2000;42(1):225–249. - PubMed
- Bremer H, Dennis PP. Modulation of Chemical Composition and Other Parameters of the Cell Growth Rate. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Washington: ASM Press; 1996. pp. 1553–1566. University of Michigan Medical School.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources