High performance computing in biology: multimillion atom simulations of nanoscale systems - PubMed (original) (raw)
Review
High performance computing in biology: multimillion atom simulations of nanoscale systems
K Y Sanbonmatsu et al. J Struct Biol. 2007 Mar.
Abstract
Computational methods have been used in biology for sequence analysis (bioinformatics), all-atom simulation (molecular dynamics and quantum calculations), and more recently for modeling biological networks (systems biology). Of these three techniques, all-atom simulation is currently the most computationally demanding, in terms of compute load, communication speed, and memory load. Breakthroughs in electrostatic force calculation and dynamic load balancing have enabled molecular dynamics simulations of large biomolecular complexes. Here, we report simulation results for the ribosome, using approximately 2.64 million atoms, the largest all-atom biomolecular simulation published to date. Several other nano-scale systems with different numbers of atoms were studied to measure the performance of the NAMD molecular dynamics simulation program on the Los Alamos National Laboratory Q Machine. We demonstrate that multimillion atom systems represent a 'sweet spot' for the NAMD code on large supercomputers. NAMD displays an unprecedented 85% parallel scaling efficiency for the ribosome system on 1024 CPUs. We also review recent targeted molecular dynamics simulations of the ribosome that prove useful for studying conformational changes of this large biomolecular complex in atomic detail.
Figures
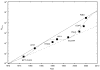
Figure 1
Increase in simulation system size with respect to year simulated. The largest bio-molecular sustainted performance simulations to date at the time of publication to our knowledge are shown. All simulations include explicit solvent unless otherwise noted. BPTI (VAC), bovine pancreatic trypsin inhibitor without solvent (McCammon 1977; Karplus and McCammon 2002); BPTI, bovine pancreatic trypsin inhibitor with solvent (Van Gunsteren and Karplus 1982); RHOD, photosynthetic reaction center of Rhodopseudomonas viridis (Heller et al. 1990); HIV-1, HIV-1 protease (Harte et al. 1992) ; ES, estrogen-DNA (Kosztin et al. 1997); STR, streptavidin (Eichinger et al. 1997); FN-III (Gao et al. 2002); DOPC, DOPC lipid bilayer (Tieleman 2004); RIBO, ribosome (Sanbonmatsu et al. 2005). Solid curve, Moore’s law doubling every 28.2 months. Dashed curve, Moore’s law doubling every 39.6 months. Dot-dashed curve, sinosoidal doubling fit (described in text).
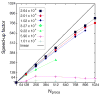
Figure 2
Performance of NAMD on the LANL Q-Machine as a function of number of atoms. Solid symbols used a cutoff of 9 Å and dt = 2 fs with SHAKE. Open symbols used a cutoff of 12 Å and dt = 1 fs without SHAKE (Phillips, et al. parameters), resulting in a factor of ~2 increase in compute load and higher parallel efficiency but longer wall clock time per step. (a) Performance measured in GFLOP/s vs. number of processors. Performance increases with increasing system size. (b) Execution time per step as a function of the number of processors.
Figure 3
Parallel performance curve. Speed-up as a function of processors for systems with different numbers of atoms. Black curve represents ideal speed-up.
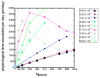
Figure 4
Physiological time simulated vs. number of processors for different numbers of atoms. The ‘turn-over’ in efficiency occurs between Natoms = 5.73x104 and 9.22x104.
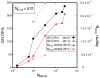
Figure 5
Performance vs. the number of atoms (black curves) and total number of atoms-ns simulated per day vs. number of atoms (red curves) for a constant number of processors (Nprocs =512). Dashed curves with open symbols use Phillips, et al. parameters.
Figure 6
Memory usage vs. number of processors for different numbers of atoms. Simulations with Natoms > 2x106 require > 2 GB RAM per processor.
Figure 7
Solvation shell of the ribosome. Cyan, water density contours at ~ 3 times the bulk density, averaged over 1 ns. White = small subunit, Green = large subunit, Pink = mRNA, Red = aminoacyl-tRNA, Yellow = peptidyl-tRNA.
Figure 8
Aminoacyl-tRNA moves from the A/T state to the A/A state during the targeted molecular dynamics simulations. Blue, oxygen atom on every 5th water molecule. White, 23S rRNA; light green, 50S ribosomal proteins; cyan, 16S rRNA; magenta, 30S ribosomal proteins; yellow, aminoacyl-tRNA; red, peptidyl-tRNA; green, mRNA. The top portion of the simulation domain is not shown in order to display the full tRNAs.
Figure 9
Entrance of the aminoacyl-tRNA 3’-CCA end (yellow) into the peptidyl transferase center of the large ribosomal subunit. Green, aminoacyl-tRNA amino acid; purple, 23S rRNA A-loop (LH92); pink, 23S rRNA LH90; blue, 23S rRNA LH89; red, universally conserved accommodation gate nucleotides; light green, peptidyl transferase center nucleotides that interact with the 3’-CCA end in the x-ray crystallography structure representing A/A state; cyan, peptidyl-tRNA amino acid.
Similar articles
- Scalable Molecular Dynamics with NAMD on the Summit System.
Acun B, Hardy DJ, Kale LV, Li K, Phillips JC, Stone JE. Acun B, et al. IBM J Res Dev. 2018 Nov-Dec;62(6):1-9. doi: 10.1147/jrd.2018.2888986. Epub 2018 Dec 21. IBM J Res Dev. 2018. PMID: 32154805 Free PMC article. - Scaling of Multimillion-Atom Biological Molecular Dynamics Simulation on a Petascale Supercomputer.
Schulz R, Lindner B, Petridis L, Smith JC. Schulz R, et al. J Chem Theory Comput. 2009 Oct 13;5(10):2798-808. doi: 10.1021/ct900292r. J Chem Theory Comput. 2009. PMID: 26631792 - Coarse-grained models to study dynamics of nanoscale biomolecules and their applications to the ribosome.
Trylska J. Trylska J. J Phys Condens Matter. 2010 Nov 17;22(45):453101. doi: 10.1088/0953-8984/22/45/453101. Epub 2010 Oct 28. J Phys Condens Matter. 2010. PMID: 21339588 Review. - Proceedings of the Second Workshop on Theory meets Industry (Erwin-Schrödinger-Institute (ESI), Vienna, Austria, 12-14 June 2007).
Hafner J. Hafner J. J Phys Condens Matter. 2008 Feb 13;20(6):060301. doi: 10.1088/0953-8984/20/06/060301. Epub 2008 Jan 24. J Phys Condens Matter. 2008. PMID: 21693862 - Molecular dynamics simulations of large macromolecular complexes.
Perilla JR, Goh BC, Cassidy CK, Liu B, Bernardi RC, Rudack T, Yu H, Wu Z, Schulten K. Perilla JR, et al. Curr Opin Struct Biol. 2015 Apr;31:64-74. doi: 10.1016/j.sbi.2015.03.007. Epub 2015 Apr 4. Curr Opin Struct Biol. 2015. PMID: 25845770 Free PMC article. Review.
Cited by
- RNA Structural Dynamics As Captured by Molecular Simulations: A Comprehensive Overview.
Šponer J, Bussi G, Krepl M, Banáš P, Bottaro S, Cunha RA, Gil-Ley A, Pinamonti G, Poblete S, Jurečka P, Walter NG, Otyepka M. Šponer J, et al. Chem Rev. 2018 Apr 25;118(8):4177-4338. doi: 10.1021/acs.chemrev.7b00427. Epub 2018 Jan 3. Chem Rev. 2018. PMID: 29297679 Free PMC article. Review. - Multiscale simulation of microbe structure and dynamics.
Joshi H, Singharoy A, Sereda YV, Cheluvaraja SC, Ortoleva PJ. Joshi H, et al. Prog Biophys Mol Biol. 2011 Oct;107(1):200-17. doi: 10.1016/j.pbiomolbio.2011.07.006. Epub 2011 Jul 23. Prog Biophys Mol Biol. 2011. PMID: 21802438 Free PMC article. - Examinations of tRNA Range of Motion Using Simulations of Cryo-EM Microscopy and X-Ray Data.
Caulfield TR, Devkota B, Rollins GC. Caulfield TR, et al. J Biophys. 2011;2011:219515. doi: 10.1155/2011/219515. Epub 2011 Mar 28. J Biophys. 2011. PMID: 21716650 Free PMC article. - Ribosome Mechanics Informs about Mechanism.
Zimmermann MT, Jia K, Jernigan RL. Zimmermann MT, et al. J Mol Biol. 2016 Feb 27;428(5 Pt A):802-810. doi: 10.1016/j.jmb.2015.12.003. Epub 2015 Dec 11. J Mol Biol. 2016. PMID: 26687034 Free PMC article. Review. - A generic force field for protein coarse-grained molecular dynamics simulation.
Gu J, Bai F, Li H, Wang X. Gu J, et al. Int J Mol Sci. 2012 Nov 8;13(11):14451-69. doi: 10.3390/ijms131114451. Int J Mol Sci. 2012. PMID: 23203075 Free PMC article.
References
- Auffinger P, Westhof E. Simulations of the molecular dynamics of nucleic acids. Current Opinion in Structural Biology. 1998;8(2):227–236. - PubMed
- Auffinger P, Westhof E. Water and ion binding around r(UpA)(12) and d(TpA)(12) oligomers: Comparison with RNA and DNA (CpG)(12) duplexes. Journal of Molecular Biology. 2001;305(5):1057–1072. - PubMed
- Auffinger P, Westhof E. Melting of the solvent structure around a RNA duplex: a molecular dynamics simulation study. Biophys Chem. 2002;95(3):203–210. - PubMed
- Board J, Causey J, Leathrum J, Windemuth A, Schulten K. Accelerated molecular dynamics simulation with the parallel fast multipole algorithm. Chemical Physics Letters. 1992;198:89–94.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources