Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes - PubMed (original) (raw)
Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes
Irfan J Lodhi et al. Cell Metab. 2007 Jan.
Abstract
Insulin stimulates glucose uptake by promoting translocation of the Glut4 glucose transporter from intracellular storage compartments to the plasma membrane. In the absence of insulin, Glut4 is retained intracellularly; the mechanism underlying this process remains uncertain. Using the TC10-interacting protein CIP4 as bait in a yeast two-hybrid screen, we cloned a RasGAP and VPS9 domain-containing protein, Gapex-5/RME-6. The VPS9 domain is a guanine nucleotide exchange factor for Rab31, a Rab5 subfamily GTPase implicated in trans-Golgi network (TGN)-to-endosome trafficking. Overexpression of Rab31 blocks insulin-stimulated Glut4 translocation, whereas knockdown of Rab31 potentiates insulin-stimulated Glut4 translocation and glucose uptake. Gapex-5 is predominantly cytosolic in untreated cells; its overexpression promotes intracellular retention of Glut4 in adipocytes. Insulin recruits the CIP4/Gapex-5 complex to the plasma membrane, thus reducing Rab31 activity and permitting Glut4 vesicles to translocate to the cell surface, where Glut4 docks and fuses to transport glucose into the cell.
Figures
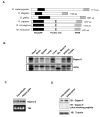
Figure 1. Gapex-5 domain structure and expression profile
A) Gapex-5 is an evolutionarily conserved protein that contains an N-terminal RasGAP domain and a C-terminal VPS9 domain, which is a Rab5 GDP/GTP exchange factor. The mammalian protein also has a central proline-rich sequence. B) Multi-tissue Northern blot analysis of Gapex-5. C) Northern blot analysis of Gapex-5 expression in 3T3-L1 fibroblasts and fully differentiated adipocytes. D) Western blot analysis of Gapex-5 expression in fibroblasts and adipocytes using a polyclonal anti-Gapex-5 antibody. The specificity of the antibody was determined using a blocking peptide.
Figure 2. Interaction of Gapex-5 with CIP4
A) Schematic of Gapex-5 mutants used in this study. B) GST-tagged Gapex-5 fragment consisting of aa 437–972 was used to pull down myc-tagged CIP4 mutants overexpressed in Cos-1 cells. C) Cos-1 cells were transiently transfected with HA-Gapex-5 and/or myc-CIP4 constructs as indicated. Whole cell lysates (upper panels) or anti-HA immunoprecipitates were subjected to immunoblotting with anti-myc or anti-HA antibodies. D) 3T3-L1 adipocytes were serum starved for 3 hr and then stimulated with or without 100 nM insulin. The cells were fixed and immuno-stained using a monoclonal antibody against CIP4. E) 3T3-L1 adipocytes were electroporated with myc-CIP4 and/or HA-Gapex-5 as indicated. After 24 hr, cells were serum starved for 3 hr and then stimulated with or without 100 nM insulin. The cells were fixed, immuno-stained and analyzed by confocal microscopy. Representative images are shown from 4 independent experiments. F) Adipocytes were transfected with HA-Gapex-5 alone (Vector) or together with myc-CIP4 mutants as described in panel 2E. The amount of HA-Gapex-5 localized at the plasma membrane as a percentage of total HA-Gapex-5 was quantified from at least 10 cells per condition per experiment using the NIH ImageJ program. The signal intensity at the plasma membrane was expressed as a percentage of total signal intensity (intracellular plus plasma membrane, arbitrary units). To ensure that the changes did not simply reflect differences in area, plasma membrane area to total cell area was measured. The data are presented as mean ± SD and is representative of two separate experiments. *Significant difference, p-value<10−7 vs. Vector (insulin).
Figure 3. Identification of Gapex-5 as a Rab GEF and EEA1 as an effector of Rab31
A) Alignment of the VPS9 domain of Gapex-5 with the VPS9 domains of known Rab5 GEFs, Rabex-5, Rin1 and Alsin. B) Lysates from Cos-1 cells overexpressing HA-Rab5a, HA-Rab31 or myc-Rab22a were incubated with GDP or GTPγS. Following pulldown with GST-tagged helical bundle/VPS9 domains of Gapex-5 (GST-HB-VPS9), the Rab proteins were detected by immunoblotting using anti-HA or anti-myc antibodies as indicated. C) GST-tagged Rab5 or Rab31 were loaded with GDP or GTPγS and incubated with lysates from Cos-1 cells. Endogenous EEA1 and Rabaptin-5 were detected by immunoblotting. D) HA-Rab5a or Rab31 overexpressed in Cos-1 cells were loaded with nucleotides and pulled down using GST-tagged N- or C-terminal Rab binding domain of EEA1 (GST-NT or GST-CT, respectively). E) HEK-293 cells were transfected with an siRNA oligonucleotide for human Gapex-5 (Gapex-5 KD) or a matching scrambled siRNA (SC). Forty eight hours later, the cells were transfected with HA-Rab31. After another 24 hrs, the cells were harvested and used in pulldown with GST-EEA1/NT. Rab31 was detected using an anti-HA antibody. Gapex-5 and CIP4 were detected using their respective antibodies. F). Rab31-GTP levels normalized to total Rab31 were quantified using the ImageJ program. The data are presented as mean ± SD of three independent experiments. *Significant difference, p-value=0.003.
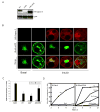
Figure 4. Gapex-5 blocks insulin-stimulated Glut4 translocation in 3T3-L1 adipocytes
A) Expression of HA-tagged Gapex-5 constructs in 3T3L1 adipocytes. Cell lysates prepared from 3T3-L1 adipocytes electroporated with full length (FL), ΔGAP or ΔHB–VPS9 constructs of HA-Gapex-5 were resolved by SDS-PAGE and immunoblotted using an anti-HA antibody. CIP4 was detected with a polyclonal anti-CIP4 antibody as a loading control. B) 3T3-L1 adipocytes were transfected with Glut4-eGFP alone (vector) or in combination with HA-tagged Gapex-5. After 24 hr, the cells were serum starved and then stimulated with or without 100 nM insulin for 30 min. The cells were fixed and then stained using a monoclonal anti-HA antibody. C) Quantification of Glut4 translocation. The cells with GFP rim staining were marked as positive for Glut4 translocation. At least 50 cells per condition per experiment were counted. The data are mean ± SD of 5 experiments. *Significant difference vs. vector (insulin), p-value ≤0.025; #Significant difference vs. WT (insulin), p-value=0.03. D) 3T3-L1 adipocytes were transfected with Glut4-eGFP/F5A alone or together with HA-Gapex-5. At 3, 6, 9 or 12 hr post-transfection, cells were serum starved for 3 hr and then stimulated with or without insulin. Plasma membrane translocation of Glut4 was quantified by determining the percentage of cells with GFP rim staining. At each time point, 50 cells per condition were counted. The data are an average of two separate experiments. The translocation of wild-type Glut4-eGFP from a representative experiment is shown for comparison.
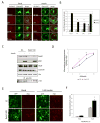
Figure 5. Plasma membrane targeting of Gapex-5 relieves its inhibitory effect on Glut4 translocation
A) 3T3-L1 adipocytes were transfected with Glut4-eGFP alone (control), Glut4-eGFP and HA-Gapex-5, or Glut4-eGFP, HA-Gapex-5 and FLAG-CIP4. After 24 hr, the cells were serum starved for 3 hr and then stimulated with or without 100 nM insulin for 30 min. The cells were fixed with paraformaldehyde and then immuno-stained using anti-HA polyclonal and anti-FLAG monoclonal antibodies. B) Quantification of the Glut4 translocation. One hundred cells per condition were counted. The data are mean ± SD of 2 separate experiments. *Significant difference vs. control (insulin), p-value=0.002. C) Schematic of Gapex-5/CAAX chimera. D) 3T3-L1 adipocytes were transfected with Glut4-eGFP alone or together with myc-Gapex-5 wild-type or its CAAX chimera. The cells were fixed and immuno-stained and analyzed by confocal microcopy. Representative images are shown. E) Quantification of Glut4 translocation. The data are mean ± SD of 3-5 separate experiments. *Significant difference vs. Control (insulin), p-value<0.03; #significant difference vs. WT (insulin), p-value<0.001.
Figure 6. Effect of Rab31 on Glut4 translocation and glucose uptake
A) 3T3-L1 adipocytes were transfected with Glut4-eGFP alone (vector) or together with HA-tagged Rab31. The cells were fixed, immuno-stained and analyzed by confocal microscopy. Representative images are shown. B) Glut4 translocation was quantified by determining the percentage of cells with GFP rim staining. At least 50 cells per condition were counted. The data are mean ± SD of 3 separate experiments. *Significant difference vs. Vector (insulin), p-value<0.02; #significant difference vs. Vector (basal), p-value=0.03. C) 3T3-L1 adipocytes were electroporated with siRNA for Rab31 (Rab31 KD) or a matching scrambled control siRNA (SC). After 72 hr, the cells were serum starved for 3 hr and then stimulated with or without 100 nM insulin. Lysates were resolved by SDS-PAGE and subjected to immunoblotting using anti-Rab31, phospho-Akt, Glut4, Caveolin and Cbl antibodies. The phosphorylation of Cbl immunoprecipitated from control and Rab31 knockdown lysates was detected by immunoblotting with phosphotyrosine 4G10 antibody. D) At 72 hr after transfection with Rab31 knockdown siRNA (Rab31 KD) or a matching scrambled oligonucleotide (SC), adipocytes were serum starved for 3 hr and then stimulated with 0, 0.1, 1, or 10 nM insulin. The uptake of 2-deoxyglucose was measured as described under Experimental Procedures. The data are mean ± SD of triplicate determinations and were reproduced five times. *Significant difference, p-value ≤ 0.025. E) Adipocytes stably infected with retrovirus expressing myc-Glut4-eGFP reporter were transfected with Rab31 knockdown or scrambled siRNA. After 72 hr, the cells were serum-starved and stimulated with or without 1 nM insulin. The cells were fixed and then immuno-stained using an anti-myc polyclonal antibody. Representative images are shown from 3 independent experiments. F) Quantification of Glut4 translocation to the plasma membrane. At least 300 cells per condition per experiment were scored for exofacial myc-Glut4 staining in two separate experiments. Data are presented as mean ± SEM. *Significant difference vs. SC (insulin), p-value<0.02.
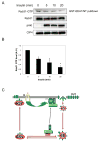
Figure 7. Insulin inhibits the activity of Rab31 in 3T3-L1 adipocytes
A) 3T3-L1 adipocytes were stimulated with 100 nM insulin for 0, 5, 10 or 20 min. The activation state of endogenous Rab31 was determined using GST-EEA1/NT as described in Experimental Procedures. B) Rab31-GTP levels normalized to total Rab31 were quantified using the ImageJ program. The data are presented as mean ± SD of three independent experiments. *Significant difference, p-value<0.03. C) A hypothetical model describing how Gapex-5 and Rab31 regulate Glut4 translocation. In the absence of insulin, Glut4 is rapidly internalized through clathrin-coated vesicles (CCV) and retained intracellularly by cycling between specialized insulin-responsive Glut4 storage vesicles (GSV) and early endosomes (EE). Gapex-5 maintains Rab31 in an active state and promotes fusion of Glut4 vesicles with endosomes, resulting in a futile cycle that retains Glut4 inside the cell. Insulin stimulation results in the activation of the Rho family GTPase TC10 and recruitment of the CIP4/Gapex-5 complex to the plasma membrane. This results in a decrease of Rab31 activity, permitting insulin responsive Glut4 vesicles to escape the futile intracellular cycle and translocate to the plasma membrane, where Glut4 docks and fuses to transport glucose into the cell. This hypothesis will require further experimentation.
Similar articles
- Insulin stimulates phosphatidylinositol 3-phosphate production via the activation of Rab5.
Lodhi IJ, Bridges D, Chiang SH, Zhang Y, Cheng A, Geletka LM, Weisman LS, Saltiel AR. Lodhi IJ, et al. Mol Biol Cell. 2008 Jul;19(7):2718-28. doi: 10.1091/mbc.e08-01-0105. Epub 2008 Apr 23. Mol Biol Cell. 2008. PMID: 18434594 Free PMC article. - The TC10-interacting protein CIP4/2 is required for insulin-stimulated Glut4 translocation in 3T3L1 adipocytes.
Chang L, Adams RD, Saltiel AR. Chang L, et al. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12835-40. doi: 10.1073/pnas.202495599. Epub 2002 Sep 19. Proc Natl Acad Sci U S A. 2002. PMID: 12242347 Free PMC article. - p75 neurotrophin receptor regulates glucose homeostasis and insulin sensitivity.
Baeza-Raja B, Li P, Le Moan N, Sachs BD, Schachtrup C, Davalos D, Vagena E, Bridges D, Kim C, Saltiel AR, Olefsky JM, Akassoglou K. Baeza-Raja B, et al. Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5838-43. doi: 10.1073/pnas.1103638109. Epub 2012 Mar 28. Proc Natl Acad Sci U S A. 2012. PMID: 22460790 Free PMC article. - Ins (endocytosis) and outs (exocytosis) of GLUT4 trafficking.
Hou JC, Pessin JE. Hou JC, et al. Curr Opin Cell Biol. 2007 Aug;19(4):466-73. doi: 10.1016/j.ceb.2007.04.018. Epub 2007 Jul 17. Curr Opin Cell Biol. 2007. PMID: 17644329 Free PMC article. Review. - Subcellular compartmentalization and trafficking of the insulin-responsive glucose transporter, GLUT4.
Watson RT, Pessin JE. Watson RT, et al. Exp Cell Res. 2001 Nov 15;271(1):75-83. doi: 10.1006/excr.2001.5375. Exp Cell Res. 2001. PMID: 11697884 Review.
Cited by
- The Vac14-interaction network is linked to regulators of the endolysosomal and autophagic pathway.
Schulze U, Vollenbröker B, Braun DA, Van Le T, Granado D, Kremerskothen J, Fränzel B, Klosowski R, Barth J, Fufezan C, Wolters DA, Pavenstädt H, Weide T. Schulze U, et al. Mol Cell Proteomics. 2014 Jun;13(6):1397-411. doi: 10.1074/mcp.M113.034108. Epub 2014 Feb 27. Mol Cell Proteomics. 2014. PMID: 24578385 Free PMC article. - Evolution of the Ras-like small GTPases and their regulators.
van Dam TJ, Bos JL, Snel B. van Dam TJ, et al. Small GTPases. 2011 Jan;2(1):4-16. doi: 10.4161/sgtp.2.1.15113. Small GTPases. 2011. PMID: 21686276 Free PMC article. - BAR domain proteins regulate Rho GTPase signaling.
Aspenström P. Aspenström P. Small GTPases. 2014;5(2):7. doi: 10.4161/sgtp.28580. Small GTPases. 2014. PMID: 25483303 Free PMC article. Review. - A Systematic Analysis of Expression and Function of RAS GTPase-Activating Proteins (RASGAPs) in Urological Cancers: A Mini-Review.
Song H, Wang G, Gao G, Xia H, Jiao L, Wu K. Song H, et al. Cancers (Basel). 2025 Apr 28;17(9):1485. doi: 10.3390/cancers17091485. Cancers (Basel). 2025. PMID: 40361412 Free PMC article. Review. - Sbf/MTMR13 coordinates PI(3)P and Rab21 regulation in endocytic control of cellular remodeling.
Jean S, Cox S, Schmidt EJ, Robinson FL, Kiger A. Jean S, et al. Mol Biol Cell. 2012 Jul;23(14):2723-40. doi: 10.1091/mbc.E12-05-0375. Epub 2012 May 30. Mol Biol Cell. 2012. PMID: 22648168 Free PMC article.
References
- Ahn MY, Katsanakis KD, Bheda F, Pillay TS. Primary and essential role of the adaptor protein APS for recruitment of both c-Cbl and its associated protein CAP in insulin signaling. J Biol Chem. 2004;279:21526–21532. - PubMed
- Aspenstrom P. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr Biol. 1997;7:479–487. - PubMed
- Bao X, Faris AE, Jang EK, Haslam RJ. Molecular cloning, bacterial expression and properties of Rab31 and Rab32. Eur J Biochem. 2002;269:259–271. - PubMed
- Baumann CA, Ribon V, Kanzaki M, Thurmond DC, Mora S, Shigematsu S, Bickel PE, Pessin JE, Saltiel AR. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. - PubMed
- Bogan JS, Hendon N, McKee AE, Tsao TS, Lodish HF. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature. 2003;425:727–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK59291/DK/NIDDK NIH HHS/United States
- R01 DK059291/DK/NIDDK NIH HHS/United States
- R01 DK061618/DK/NIDDK NIH HHS/United States
- 5P60-DK20572/DK/NIDDK NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- R01-DK061618/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous