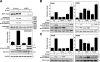Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress - PubMed (original) (raw)
Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress
Anna Zagórska et al. J Cell Biol. 2007.
Abstract
Mutations within the WNK1 (with-no-K[Lys] kinase-1) gene cause Gordon's hypertension syndrome. Little is known about how WNK1 is regulated. We demonstrate that WNK1 is rapidly activated and phosphorylated at multiple residues after exposure of cells to hyperosmotic conditions and that activation is mediated by the phosphorylation of its T-loop Ser382 residue, possibly triggered by a transautophosphorylation reaction. Activation of WNK1 coincides with the phosphorylation and activation of two WNK1 substrates, namely, the protein kinases STE20/SPS1-related proline alanine-rich kinase (SPAK) and oxidative stress response kinase-1 (OSR1). Small interfering RNA depletion of WNK1 impairs SPAK/OSR1 activity and phosphorylation of residues targeted by WNK1. Hyperosmotic stress induces rapid redistribution of WNK1 from the cytosol to vesicular structures that may comprise trans-Golgi network (TGN)/recycling endosomes, as they display rapid movement, colocalize with clathrin, adaptor protein complex 1 (AP-1), and TGN46, but not the AP-2 plasma membrane-coated pit marker nor the endosomal markers EEA1, Hrs, and LAMP1. Mutational analysis suggests that the WNK1 C-terminal noncatalytic domain mediates vesicle localization. Our observations shed light on the mechanism by which WNK1 is regulated by hyperosmotic stress.
Figures
Figure 1.
Characterization of WNK1 activity. (A) 293 cells were stimulated with 0.5 M sorbitol for 30 min. Immunoprecipitations from the cell lysates were performed with the anti-WNK1(CT) antibody (WNK1-Ab) or the preimmune antibody (PI-Ab), and the kinase activity of the immunoprecipitates was measured using either kinase-inactive OSR1[D164A] (ki-OSR1) or a mutant of OSR1 in which the WNK1 phosphorylation sites have been changed to Ala (ki-OSR1[T185A,S325A]). Phosphorylation of OSR1 was analyzed after electrophoresis on a polyacrylamide gel, Coomassie blue staining (middle), and autoradiography (top). The incorporation of phosphate was also quantified on a Wallac Scintillation Counter (graph), and results are presented as the mean specific activity ± SD for duplicate samples. Similar results were obtained in at least two experiments. (B) As above, except that 293 cells were stimulated with the indicated concentrations of sorbitol for 30 min. (C) As in A, except that 293 cells were stimulated with 0.5 M sorbitol for the indicated times. (D) As in B, except that 293 cells were stimulated with the indicated concentrations of NaCl for 30 min. (E) As in B, except that 293 cells were deprived of serum for 16 h and treated with the indicated stimuli for 30 min. For the hypotonic stimulation, the cell medium was diluted twofold in H2O. Cell lysates were also immunoblotted with the indicated antibodies. (F) As in E, except that cells were preincubated with 2 μM PD184352 for 30 min before stimulation with 0.5 M sorbitol for 30 min. (G) As in E, except that cells were either left untreated (−) or preincubated (+) with 10 μM SB203580 or the indicated concentrations of BIRB0796 for 30 min and then stimulated with 10 μg/ml anisomycin or 0.5 M sorbitol for an additional 30 min (in the continued absence or presence of inhibitor).
Figure 2.
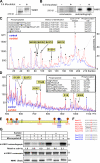
Identification of phosphorylation sites on WNK1. (A) Endogenous WNK1 was immunoprecipitated from control or sorbitol-stimulated (0.5 M for 30 min) 293 cells and subjected to immunoblot analysis with the anti-WNK1 antibody. (B) 293 cells were labeled with 32P and left untreated or stimulated with 0.5 M sorbitol for 20 min. The endogenously expressed WNK1 was immunoprecipitated and subjected to electrophoresis on a polyacrylamide gel, which was then stained with colloidal Coomassie blue and autoradiographed. The Coomassie-stained bands migrating with the expected molecular mass of WNK1 were excised from the gel and digested with trypsin, and a small aliquot (<1%) was analyzed by MALDI TOF/TOF mass spectrometry and confirmed to contain WNK1 tryptic peptides. (C) The remaining WNK1 tryptic peptides were chromatographed on a reverse-phase HPLC Vydac C18 column. The figure shows 32P radioactivity versus HPLC fraction number for the phosphopeptides derived from WNK1 isolated from control (blue) or sorbitol-stimulated (red) cells. A similar HPLC profile was obtained in two separate experiments. All major 32P-labeled peptides were analyzed by MALDI TOF/TOF and 4000 Q-TRAP mass spectrometry. This resulted in the identification of five phosphopeptides indicated by the yellow boxes, and the sites of phosphorylation of the phosphopeptides identified by MALDI TOF/TOF were confirmed by solid-phase Edman sequencing (Ed. seq.). There is mass spectrometry evidence that the peptide labeled with a solid square is a diphospho form of the DVDDGSGSPHSPHQLSSK peptide that may be labeled at both Ser2029 and Ser2032. (D) WNK1 was immunoprecipitated from 100 mg of unlabeled 293 cell lysate derived from control or sorbitol-stimulated (0.5 M, for 30 min) cells. The samples were subjected to electrophoresis on a polyacrylamide, and the Coomassie-stained bands corresponding to WNK1 were excised and digested with trypsin. Phosphopeptides were identified by combined liquid chromatography–mass spectrometry and tandem mass spectrometry analysis. The figure shows the signal intensity (cps, counts of ions per second detected) versus the ion distribution (m/z) for the phosphopeptides derived from WNK1 isolated from control (blue) or sorbitol-stimulated (red) cells. We were unable to identify the peptides marked with asterisks. (E) Illustration of the location of the identified sorbitol-induced (red) and constitutive (blue) sites of phosphorylation within the WNK1 protein. KD, kinase domain (residues 221–479); CC, coiled-coil domains (residues 189–221, 566–595, and 2072–2101). (F) The amino acid sequence surrounding each of the phosphorylation sites identified in human WNK1 is presented. The RFXV/I motif lying adjacent to the site of phosphorylation of Ser1261 is underlined. (G) WNK1 immunoprecipitates derived from control and sorbitol-stimulated (0.5 M, 30 min) 293 cells were incubated with PP1γ, in the presence or absence of microcystin-LR, an inhibitor of Ser/Thr protein phosphatases, and assayed as described in the legend to Fig. 1. The results are presented as relative activity compared with that of WNK1 isolated from untreated cells and not treated with PP1γ, which was given a value of 1.0. An aliquot of the reaction mixtures was also immunoblotted with the anti-WNK1 antibody. Similar results were obtained in two separate experiments conducted in duplicate.
Figure 3.
Evidence that phosphorylation of Ser382 mediates WNK1 activation. (A) 293 cells were transfected with constructs encoding the indicated forms of GST-WNK1[1–667]. 36 h after transfection, the cells were left untreated or treated with 0.5 M sorbitol for 30 min and lysed. GST-WNK1 was affinity purified on glutathione–Sepharose and assayed as described in the legend to Fig. 1. The results are presented as the mean relative activity ± SD compared with the activity observed for wild-type WNK1[1–667] isolated from untreated cells, which is given a value of 1.0 (its specific activity was 0.13 U/mg). Similar results were obtained in at least two experiments conducted in duplicate. Cell lysates were immunoblotted with the indicated antibodies. (B) The indicated forms GST-WNK1[1–661] were expressed in E. coli, purified, and assayed. Results are presented as the mean specific activity for duplicate samples, and similar results were obtained in at least two experiments. An aliquot of the reaction mixtures was also immunoblotted with the anti-WNK1 antibody. The WNK1[1–661], WNK1[S382E, 1–661], or WNK1[S382D, 1–661] fragments migrate for unknown reasons as a doublet band on SDS-PAGE, whereas the WNK1[D368A, 1–661] or WNK1[S382A, 1–661] migrate as a single band with the expected molecular mass of 77 kD. The question mark indicates that the identity of the slower migrating WNK1 band is uncertain. (C) The indicated forms of WNK1 purified from E. coli were incubated in the absence (−) or presence (+) of 0.1 mM ATP and 10 mM Mg for 1 h before immunoblotting with the indicated antibodies.
Figure 4.
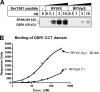
Evidence that phosphorylation of Ser1261 inhibits binding of WNK1 to SPAK/OSR1. (A) The indicated amounts of a biotinylated peptide encompassing Ser1261 of WNK1 (biotin-AGRRFIVSPVPESRL) or the phosphorylated form of this peptide (AGRRFIVpSPVPESRL) were conjugated to streptavidin–Sepharose and incubated with 1 mg of 293 cell lysate. After isolation and washes of the beads, the samples were electrophoresed on a polyacrylamide gel and immunoblotted with an antibody recognizing both SPAK and OSR1. (B) The binding of bacterially expressed purified forms of the OSR1 CCT domain (residues 429–527) to the biotinylated peptides used above was analyzed using BiaCore. The analysis was performed over a range of protein concentrations (6.8–340 nM), and the response level at steady state was plotted against the log of the protein concentration. The dissociation constant was calculated by fitting the data to the formula (Y = Bmax*X/[K d +X]) using GraphPad 4 software, which describes the binding of a ligand to a receptor that follows the law of mass action. Bmax is the maximal binding, and K d is the concentration of ligand required to reach half-maximal binding. X and Y correspond to the protein concentration and the response units, respectively. The asterisk indicates that binding to the phosphorylated peptide was too weak to reliably calculate a dissociation constant.
Figure 5.
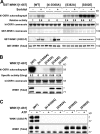
Hyperosmotic stress leads to WNK1-mediated activation and phosphorylation of SPAK and OSR1. (A) HeLa cells were transfected with a control siRNA duplex or a siRNA duplex targeting human WNK1 as described in Materials and methods. 48 h after transfection, cells were either left untreated or stimulated with 0.5 M sorbitol for 30 min. Endogenously expressed SPAK and OSR1 were immunoprecipitated from the cell extracts using an antibody that immunoprecipitates both SPAK and OSR1, and their activity was assayed using the CATCHtide peptide substrate (Vitari et al., 2006). Results are presented as the mean relative activity ± SD compared with that observed for SPAK/OSR1 isolated from untreated cells that have been transfected with the control siRNA duplex, which was given a value of 1.0. Total cell extracts (20 μg) were also immunoblotted with the indicated antibodies. Similar results were obtained in three separate experiments conducted in duplicate. (B and C, top) 293 cells were transfected with constructs encoding the wild-type and indicated mutant forms of GST-OSR1 (B) or GST-SPAK (C). 36 h after transfection, the cells were left untreated or treated with 0.5 M sorbitol for 30 min and lysed. GST-OSR1/SPAK forms were affinity purified on glutathione–Sepharose and assayed using a fragment of NKCC1 encompassing residues 1–260. Results are presented as the mean relative activity ± SD compared with the activity observed for wild-type OSR1/SPAK isolated from untreated cells, which is given a value of 1.0. Similar results were obtained in at least two experiments conducted in triplicate. (bottom) The cell lysates were also immunoblotted with the indicated phosphospecific antibodies as well as anti-GST antibody to detect GST-OSR1/SPAK proteins.
Figure 6.
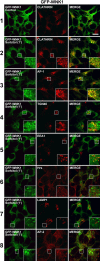
Translocation of GFP-WNK1 to clathrin-coated vesicles after hyperosmotic stress. 293 cells stably expressing GFP-WNK1 were left unstimulated (control) or stimulated with 0.2 M sorbitol for 1 min, before fixation. Cells were immunostained in the red channel with antibodies recognizing clathrin, AP-1, TGN46, EEA1, Hrs, LAMP1, or AP-2 and in the green channel for GFP-WNK1. Fluorescent imaging was performed on a confocal microscope. Similar results were obtained in two independent experiments. Bar, 10 μm.
Figure 7.
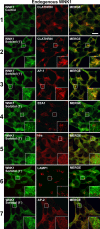
Translocation of endogenous WNK1 to clathrin-coated vesicles after hyperosmotic stress. 293 cells were left unstimulated (control) or stimulated with 0.2 M sorbitol for 1 min, before fixation. Cells were immunostained in the red channel with antibodies recognizing clathrin, AP-1, EEA1, Hrs, LAMP1, or AP-2 and in the green channel with the anti-WNK1 antibody to detect endogenous WNK1. Fluorescent imaging was performed on a confocal microscope. Similar results were obtained in two independent experiments. Bar, 10 μm.
Figure 8.
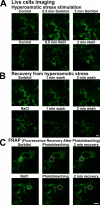
Localization of GFP-WNK1 in living cells. (A) 293 cells stably expressing GFP-WNK1 were mounted in a heated chamber for live-cell fluorescent imaging using a confocal microscope. Images were collected every 10 s. Before stimulation, cells were visualized for 2 min, and the cells labeled control correspond to a representative image of the cells during this period. After 2 min, the medium was exchanged with prewarmed medium containing either 0.2 M sorbitol (top) or 0.2 M NaCl (bottom), and the cells were observed for a further 5 min. An image of the cells after 0.5 and 2 min is shown. (B) As described in A, except that after imaging the sorbitol (top) or NaCl-treated (bottom) cells for 5 min, the medium was exchanged twice with prewarmed DME not containing sorbitol or NaCl and the cells were imaged for a further 5 min. The image of the cells after 1 and 2 min is shown. (C) As in A, except that the region indicated with the dotted circle was photobleached and cells were observed for a further 5 min, imaging every 10 s. The image of the cells after 2 min is shown. The photobleaching experiment was performed in duplicate, and at least four separate cells were visualized in each experiment. A representative cell from these experiments is shown. Bars, 10 μm.
Figure 9.
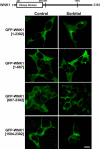
The C-terminal noncatalytic region of WNK1 mediates translocation to intracellular vesicles. 293 cells were transfected with the indicated constructs encoding GFP-tagged WNK1. 24 h after transfection, the cells were left untreated or treated with 0.2 M sorbitol for 5 min and analyzed for cell fluorescent imaging using a confocal microscope. Similar results were obtained in two independent experiments. Bar, 10 μm.
Similar articles
- The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases.
Vitari AC, Deak M, Morrice NA, Alessi DR. Vitari AC, et al. Biochem J. 2005 Oct 1;391(Pt 1):17-24. doi: 10.1042/BJ20051180. Biochem J. 2005. PMID: 16083423 Free PMC article. - SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation.
Thastrup JO, Rafiqi FH, Vitari AC, Pozo-Guisado E, Deak M, Mehellou Y, Alessi DR. Thastrup JO, et al. Biochem J. 2012 Jan 1;441(1):325-37. doi: 10.1042/BJ20111879. Biochem J. 2012. PMID: 22032326 Free PMC article. - Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1.
Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Vitari AC, et al. Biochem J. 2006 Jul 1;397(1):223-31. doi: 10.1042/BJ20060220. Biochem J. 2006. PMID: 16669787 Free PMC article. - The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters.
Alessi DR, Zhang J, Khanna A, Hochdörfer T, Shang Y, Kahle KT. Alessi DR, et al. Sci Signal. 2014 Jul 15;7(334):re3. doi: 10.1126/scisignal.2005365. Sci Signal. 2014. PMID: 25028718 Review. - Kelch-like 3/Cullin 3 ubiquitin ligase complex and WNK signaling in salt-sensitive hypertension and electrolyte disorder.
Sohara E, Uchida S. Sohara E, et al. Nephrol Dial Transplant. 2016 Sep;31(9):1417-24. doi: 10.1093/ndt/gfv259. Epub 2015 Jul 6. Nephrol Dial Transplant. 2016. PMID: 26152401 Review.
Cited by
- Protein kinase WNK1 promotes cell surface expression of glucose transporter GLUT1 by regulating a Tre-2/USP6-BUB2-Cdc16 domain family member 4 (TBC1D4)-Rab8A complex.
Mendes AI, Matos P, Moniz S, Jordan P. Mendes AI, et al. J Biol Chem. 2010 Dec 10;285(50):39117-26. doi: 10.1074/jbc.M110.159418. Epub 2010 Oct 11. J Biol Chem. 2010. PMID: 20937822 Free PMC article. - Regulation of erythrocyte Na+/K+/2Cl- cotransport by an oxygen-switched kinase cascade.
Zheng S, Krump NA, McKenna MM, Li YH, Hannemann A, Garrett LJ, Gibson JS, Bodine DM, Low PS. Zheng S, et al. J Biol Chem. 2019 Feb 15;294(7):2519-2528. doi: 10.1074/jbc.RA118.006393. Epub 2018 Dec 18. J Biol Chem. 2019. PMID: 30563844 Free PMC article. - Characterization of a novel phosphorylation site in the sodium-chloride cotransporter, NCC.
Rosenbaek LL, Assentoft M, Pedersen NB, MacAulay N, Fenton RA. Rosenbaek LL, et al. J Physiol. 2012 Dec 1;590(23):6121-39. doi: 10.1113/jphysiol.2012.240986. Epub 2012 Sep 10. J Physiol. 2012. PMID: 22966159 Free PMC article. - WNK1, a molecular crowding sensor, links phase separation to cellular physiological stress.
Lan T, Zhou F, Zhang L. Lan T, et al. MedComm (2020). 2023 Mar 18;4(2):e232. doi: 10.1002/mco2.232. eCollection 2023 Apr. MedComm (2020). 2023. PMID: 36941827 Free PMC article. No abstract available. - Emerging roles for WNK kinases in cancer.
Moniz S, Jordan P. Moniz S, et al. Cell Mol Life Sci. 2010 Apr;67(8):1265-76. doi: 10.1007/s00018-010-0261-6. Epub 2010 Jan 22. Cell Mol Life Sci. 2010. PMID: 20094755 Free PMC article. Review.
References
- Chen, W., M. Yazicioglu, and M.H. Cobb. 2004. Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J. Biol. Chem. 279:11129–11136. - PubMed
- Cope, G., A. Golbang, and K.M. O'Shaughnessy. 2005. WNK kinases and the control of blood pressure. Pharmacol. Ther. 106:221–231. - PubMed
- Darman, R.B., and B. Forbush. 2002. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J. Biol. Chem. 277:37542–37550. - PubMed
- Delaloy, C., J. Hadchouel, and X. Jeunemaitre. 2005. With-no-lysine kinases: the discovery of a new pathway in hypertension using human genetic studies. Hypertension. 46:263–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous