Monocytes give rise to mucosal, but not splenic, conventional dendritic cells - PubMed (original) (raw)
Monocytes give rise to mucosal, but not splenic, conventional dendritic cells
Chen Varol et al. J Exp Med. 2007.
Abstract
The mononuclear phagocyte (MP) system is a body-wide macrophage (MPhi) and dendritic cell (DC) network, which contributes to tissue homeostasis, inflammation, and immune defense. The in vivo origins of MPs remain poorly understood. Here, we use an adoptive precursor cell transfer strategy into MP-depleted mice to establish the in vivo differentiation sequence from a recently identified MPhi/DC-restricted bone marrow (BM) precursor (MDP) via BM and blood intermediates to peripheral MPhis and DCs. We show that MDPs are in vivo precursors of BM and blood monocytes. Interestingly, grafted Gr1high "inflammatory" blood monocytes shuttle back to the BM in the absence of inflammation, convert into Gr1low monocytes, and contribute further to MP generation. The grafted monocytes give rise to DCs in the intestinal lamina propria and lung, but not to conventional CD11chigh DCs in the spleen, which develop during homeostasis from MDPs without a monocytic intermediate.
Figures
Figure 1.
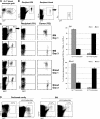
Characterization of CX3CR1gfp BM and IBC transfer of MDPs. (A) Flow cytometry analysis of CX3CR1gfp/+ BM. The cells in the dot plots to the right are gated according to CD115 and GFP expression. Note the presence of three main CD115+ GFP+ populations: CD11b− CD117+ MDP, CD11b+ Gr1high, and CD11b+ Gr1low BM monocytes. (B) Flow cytometry analysis of an MDP graft isolated by high speed sorting according to CD117 and GFP expression. (C) Analysis of WT recipient BM (right, injected femur) at the indicated time points after IBC transfer of 2.5 × 104 MDPs (purity: 85%, devoid of Gr1high CD11b+ cells). Note the distinct GFP intensity pattern of differentiated graft-derived (CD45.1+) CD11b+ Gr1high and Gr1low BM monocytes and the decrease in the Gr1high/Gr1low ratio with time: 14 (day 3) to 2.7 (day 6). (D) Analysis of WT recipient BM (left, noninjected femur) at the indicated time points after IBC transfer. Gating as in C. (E) Analysis of recipient blood (day 6). The cells were gated according to CD115 surface marker expression. The data are representative of at least two independent experiments per time point.
Figure 2.
IBC transfer of BM monocytes. (A) Flow cytometry analysis of Gr1high BM monocyte graft. (B) Analysis of WT recipient BM (right, injected femur) at the indicated time points after IBC transfer of 5 × 105 Gr1high BM monocytes (purity: 90%). Note the distinct GFP intensity pattern of differentiated graft-derived (CD45.1+) CD11b+ Gr1high and Gr1low cells and the decrease in the Gr1high/Gr1low ratio with time: 5 (day 1) to 1.8 (day 3). (C) Analysis of WT recipient BM (left, noninjected femur) at the indicated time points after IBC transfer. Gating as in C. The data are representative of at least two independent experiments per time point. (D) Analysis of recipient blood (day 3). Note the sizeable presence of graft-derived (CD45.1+) CD11b+ Gr1low monocytes.
Figure 3.
Gr1high blood and BM monocytes shuttle between blood and BM. (A) Flow cytometry analysis of a blood monocyte graft isolated from Rag−/− CX3CR1gfp mice. The GFP− cells are Ly6G+ CX3CR1− granulocytes (reference 18). (B) Analysis of recipient BM and blood 4 d after adoptive cell transfer of Gr1high blood monocytes (105 cells). Note the presence of graft-derived (GFP+) Gr1low monocytes in the blood. Fractions of graft-derived GFP+ cells of total CD115+ cells in recipient BM (0.12%) and in recipient blood (0.76%). (C) Analysis of BM and blood of WT recipients of Gr1high BM monocytes (5 × 105 cells; purity: 96%) days 1 and 3 after i.v. transfer. Note the loss of Ly6C/G expression on grafted Gr1high BM monocytes. Bar diagrams summarize data obtained from three mice per time point. Note the difference of GFP intensity of BM and blood monocytes on day 1 (mean fluorescence intensity: 274.75 ± 13.9 vs. 376.6 ± 20.5; P = 0.003). (D) Flow cytometry analysis of BM and peritoneal cavity (PC) lavage of WT recipients of MACS-purified CD115+ BM monocytes (106 cells; purity: 88%) that were left untreated or had been inoculated with thioglycollate. Note the recruitment of grafted Gr1high BM monocytes to the inflamed peritoneal cavity. The data are representative of at least two independent experiments per time point.
Figure 4.
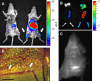
Whole body imaging of Gr1high BM monocyte recipients. (A) Color-coded near-infrared fluorescent image overlaid on a photographic image of a CD1 nude mouse 16 h after tail vein injection of DiR-labeled BM monocytes (3 × 106 cells; purity: 94%; right). Control (acquired on IVIS 100) is shown on the left. Note the presence of label in femura. (B) Color-coded near-infrared fluorescent image overlaid on a photographic image of isolated lung, spleen, and femura of CD1 nude recipients of DiR-labeled BM monocytes (right) and noninjected control (left; 3 × 106 cells; purity: 94%). (C) Monochrome fluorescent microscopy image of a cranium of a CD1 nude mouse that received DiR-labeled BM monocytes (3 × 106 cells; purity: 94%). Note the presence of labeled cells in bone cavities. (D) Dual-channel fluorescent microscopic image of a cranium of a C57BL/6 WT recipient mouse that had received an i.v. injection of CFSE-labeled BM monocytes (3 × 106 cells; purity: 96%). Arrows indicate two graft-derived cells that extravasated into the BM parenchyma. Bar, 100 μm. The data are representative of two independent experiments.
Figure 5.
MDPs, but not monocytes, act as precursors of splenic DCs. (A) Flow cytometry analysis of CX3CR1gfp donor spleen and spleens of DTx-treated, MP-depleted mice with and without engraftment of CX3CR1gfp MDP (8 × 104 cKit+ CD115+ Gr1− CD11b− cells; purity: 80%) and Gr1high BM monocytes (8 × 105 CD115+ Gr1+ CD11b+ cells; purity: 88%). Note the presence of graft-derived (CD45.1+) DCs (CD11chigh) in MDP recipients that, as in donor mice CD11b− DCs, are split into CX3CR1/GFP+ and CX3CR1/GFP− cells. CD11chigh/CD11cint ratios in an MDP recipient: 2.7 in Gr1high monocyte recipient 0.05 (as determined by the indicated gates). (B) Flow cytometry analysis of the spleen of an MP-depleted mouse with and without intra-splenic injection of CX3CR1gfp BM MDPs (2.5 × 104 cells; purity: 96%). Note the presence of graft-derived (CD45.1+) DCs (CD11chigh) in MDP recipients. The data are representative of two independent experiments.
Figure 6.
Monocytes are precursors of MPs in the intestine and lung. (A) Flow cytometry analysis of small intestinal lp cells isolated from a CX3CR1gfp donor mouse, as well as of WT, MP-depleted, and MP-deficient recipients of CX3CR1gfp Gr1high BM monocytes and MDPs (7 d after i.v. transfer of 8 × 105 CD115+ CD11b+ Gr1+ monocytes [purity: 88%] or 8 × 104 CD117+ Gr1− CD11b− MDPs [purity: 80%]). Note the presence of graft-derived (CD45.1+) CD11c+ CX3CR1/GFP+ lpDCs and CD11c+ CX3CR1/GFP− lpMΦs in donors and MP-depleted and MP-deficient recipients of precursor grafts. The data are representative of three independent experiments. (B) Fluorescence microscopy analysis of small intestinal villi of mice constitutively lacking lpDCs and lpMΦs with or without adoptive transfer of CX3CR1gfp BM monocytes (106 CD115+ Gr1high CD11b+ cells; purity: 85%). Note the presence of graft-derived CX3CR1/GFP+ lpDCs in the live tissue of a BM monocyte recipient. Bar, 100 μm. The data are representative of three independent experiments. (C) Flow cytometry analysis of parenchymal lung cells isolated from CX3CR1gfp donor mouse, an MP-depleted mouse, and MP-depleted recipients of CX3CR1gfp Gr1high BM monocytes (7 d after i.v. transfer of 7.5 × 105 CD115+ Gr1+ CD11b+ cells; purity: 85%). Note the presence of graft-derived (CD45.1+) CD11c+ CD11b+ CX3CR1/GFP+ lung DCs in the graft recipient. The data are representative of two independent experiments.
Similar articles
- Intestinal lamina propria dendritic cell subsets have different origin and functions.
Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Varol C, et al. Immunity. 2009 Sep 18;31(3):502-12. doi: 10.1016/j.immuni.2009.06.025. Epub 2009 Sep 3. Immunity. 2009. PMID: 19733097 - Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8- and CD8+ splenic dendritic cells.
León B, Martínez del Hoyo G, Parrillas V, Vargas HH, Sánchez-Mateos P, Longo N, López-Bravo M, Ardavín C. León B, et al. Blood. 2004 Apr 1;103(7):2668-76. doi: 10.1182/blood-2003-01-0286. Epub 2003 Nov 20. Blood. 2004. PMID: 14630812 - Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon.
Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Rivollier A, et al. J Exp Med. 2012 Jan 16;209(1):139-55. doi: 10.1084/jem.20101387. Epub 2012 Jan 9. J Exp Med. 2012. PMID: 22231304 Free PMC article. - Dendritic cell subsets in the intestinal lamina propria: ontogeny and function.
Persson EK, Scott CL, Mowat AM, Agace WW. Persson EK, et al. Eur J Immunol. 2013 Dec;43(12):3098-107. doi: 10.1002/eji.201343740. Epub 2013 Aug 21. Eur J Immunol. 2013. PMID: 23966272 Free PMC article. Review. - Inflammatory dendritic cells in mice and humans.
Segura E, Amigorena S. Segura E, et al. Trends Immunol. 2013 Sep;34(9):440-5. doi: 10.1016/j.it.2013.06.001. Epub 2013 Jul 4. Trends Immunol. 2013. PMID: 23831267 Review.
Cited by
- Unravelling monocyte functions: from the guardians of health to the regulators of disease.
Mildner A, Kim KW, Yona S. Mildner A, et al. Discov Immunol. 2024 Aug 30;3(1):kyae014. doi: 10.1093/discim/kyae014. eCollection 2024. Discov Immunol. 2024. PMID: 39430099 Free PMC article. Review. - Causal role of immune cells in IgA nephropathy: a mendelian randomization study.
Shu J, Ge Y, Wu Y. Shu J, et al. Ren Fail. 2024 Dec;46(2):2381593. doi: 10.1080/0886022X.2024.2381593. Epub 2024 Jul 22. Ren Fail. 2024. PMID: 39039855 Free PMC article. - Monocyte-derived dendritic cells can be detected in urine of kidney transplant recipients with pathogenic asymptomatic bacteriuria.
Salvadé V, Manuel O, Golshayan D, Obregon C. Salvadé V, et al. Front Transplant. 2024 Jun 12;3:1366104. doi: 10.3389/frtra.2024.1366104. eCollection 2024. Front Transplant. 2024. PMID: 38993772 Free PMC article. - Origin and Function of Monocytes in Inflammatory Bowel Disease.
Liao X, Liu J, Guo X, Meng R, Zhang W, Zhou J, Xie X, Zhou H. Liao X, et al. J Inflamm Res. 2024 May 13;17:2897-2914. doi: 10.2147/JIR.S450801. eCollection 2024. J Inflamm Res. 2024. PMID: 38764499 Free PMC article. Review. - Transcription factor C/EBPα is required for the development of Ly6Chi monocytes but not Ly6Clo monocytes.
Kim S, Chen J, Ou F, Liu TT, Jo S, Gillanders WE, Murphy TL, Murphy KM. Kim S, et al. Proc Natl Acad Sci U S A. 2024 Apr 9;121(15):e2315659121. doi: 10.1073/pnas.2315659121. Epub 2024 Apr 2. Proc Natl Acad Sci U S A. 2024. PMID: 38564635 Free PMC article.
References
- Metchnikoff, E. 1887. Ueber den Kampf der Zellen gegen Erypselkokken, ein Beitrag zur Phagocytenlehre. Arch. Pathol. Anat. (Virchow's Archive) 107:209–249.
- Taylor, P.R., L. Martinez-Pomares, M. Stacey, H.H. Lin, G.D. Brown, and S. Gordon. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23:901–944. - PubMed
- Shortman, K., and Y.J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161. - PubMed
- Godin, I., and A. Cumano. 2002. The hare and the tortoise: an embryonic haematopoietic race. Nat. Rev. Immunol. 2:593–604. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases