Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes - PubMed (original) (raw)
Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes
E G Pamer et al. Nature. 1991.
Abstract
Listeria monocytogenes is a Gram-positive bacterium which grows in the cytoplasm of eukaryotic cells and can cause severe disease in immunocompromised individuals. In murine systems CD8+ T lymphocytes have been shown to be important effectors of acquired protective immunity against L. monocytogenes. Class I MHC-restricted CD8+ cytotoxic T lymphocytes (CTL), which lyse J774 macrophage-like targets infected with L. monocytogenes, are induced following in vivo injection of live organisms. Natural peptide epitopes derived from L. monocytogenes can be acid-extracted from heavily infected BALB/c spleens and detected by CTL. A CTL clone, B9, derived from a (BALB/c x C57BL/6)F1 (H-2dxb) mouse, recognizes one of these natural epitopes in an H-2Kd-restricted fashion. B9 also recognizes P815 (H-2d) mastocytoma cells transfected with the listeriolysin gene. To identify the region of the listeriolysin recognized by CTL we used the H-2Kd peptide-binding motif described by Rammensee and colleagues to synthesize 11 nonamer peptides. One of these peptides, listeriolysin 91-99, was recognized very efficiently by B9. This represents the first identified class I MHC-restricted epitope of bacteria and demonstrates the utility of the allele-specific motif for predicting CTL epitopes.
Figures
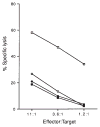
FIG. 1
CTL lysis of J774 cells infected with L. monocytogenes. J774 macrophages were treated with virulent L. monocytogenes (□), avirulent L. monocytogenes (○), heat-killed virulent L. monocytogenes (●) or left untreated (■). Effectors were added to 51Cr-labelled targets at the ratios indicated and percentage 51Cr release was determined as described. METHODS. CTL line LmT was obtained from a (BALB/c × C57BL/6) F1 mouse 8 days after infection with 5 × 103 virulent L. monocytogenes (ATCC 43251). Flasks for in vitro stimulation were prepared by adding 5 × 106 J774 cells in 10 ml RP10 lacking antibiotics. After 24 h the flasks were infected with 1 ml virulent L. monocytogenes in mid-log phase (_A_600 of 0.1) for 25 min, at which time the medium was replaced with RP10 containing 5μg ml−1 gentamicin. Three hours later medium was replaced with RP10 containing 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 50 μg ml−1 gentamicin and incubated overnight. Immune splenocytes (3×107) were added to the flasks (in 20 ml volume) and incubated upright. CTL were restimulated every 7–12 days and after the first two stimulations the media was suplemented with interleukin-2. CTL were assayed by labelling 106 J774 cells with 100 μCi 51Cr for 45 min in antibiotic-free media. Cells (104) were placed into wells and allowed to adhere for 30 min. Cells were then exposed to either 2 × 106 live or heat-killed (60 °C for 30 min) virulent or avirulent (ATCC 43250) L. monocytogenes for 25 min. Medium was replaced with RP10 containing 5 μg ml−1 gentamicin, CTL were added and lysis was allowed to proceed for 3 h. Spontaneous lysis of all target cells was <30%.
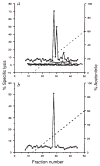
FIG. 2
Recognition by CTL line LmT and clone B9 of natural peptides derived from L. monocytogenes by acid extraction of infected spleens, a, CTL line LmT was used to lyse 51Cr-labelled P815 cells coated with reversed-phase HPLC fractions of acid extracts from infected BALB/c (■) and C57BL/6 (○) spleens and uninfected BALB/c (□) spleens. EL4 cells were also used with HPLC fractions from infected BALB/c spleens (●). Effector to target ratio was 20:1. b, CTL clone B9 was assayed on P815 cells coated with HPLC fractions of acid extracts from infected BALB/c spleens (△). Effector to target ratio was 20:1. METHODS. BALB/c mice were infected with 5×105 L. monocytogenes and C57BL/6 mice were infected with 106 L. monocytogenes. After 48 h the spleens were taken and homogenized sequentially with a tissue grinder and dounce homogenizer in 0.1% trifluoroacetic acid (TFA) and sonicated as described. This material was centrifuged at 100,000g for 30 min and the supernatant was passed over a Sephadex G-25 column. Material of less than 5K was collected and lyophilized. HPLC of the acid extract from one spleen in 0.1% TFA was performed with a C18 300A reversed-phase column using a 0–60% gradient of acetonitrile with 0.1% TFA at a rate of 1 ml min−1 and 1 ml fractions were collected. Fractions were lyophilized and suspended in 100 μl PBS. CTL assays were performed for 4 h with LmT and B9 effectors using either P815 or EL4 target cells in 200-μ1 wells, of which 50 μl represented the HPLC fraction.
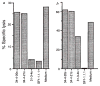
FIG. 3
Presentation of HPLC Fraction 28 by H-2Kd and lysis of P815 cells transfected with LLO. a, P815 cells were coated with HPLC fraction 28 peptides and exposed to LmT CTL in the presence of monoclonal antibodies 34-4-20s and 34-4-21s, which are specific for H-2Da, and 31-3-4s and SF1-1.1.1, which are specific for H-2Kd. b, P815 cells transfected with LLO (PHem3) were used as targets for LmT CTL in the presence and absence of anti-H-2d antibodies as in a. METHODS. P815 and PHem3 cells were labelled with 51Cr and suspended in culture supernatants from hybridomas 34-4-20s, 34-4-21s, 31-3-4s, SF1-1.1.1 and control medium. Fraction 28 peptides were added to the P815 targets. LmT effectors were added to targets at a ratio of 20:1 and the specific lysis was determined after 3 h. PHem3 was obtained by transfecting P815 cells with linearized pH_β_APr-1-neo containing the entire LLO gene. Bases 1-1,587 of the hlyA gene encoding LLO were cloned into the _Bam_HI site of the pH_β_APr-1-neo expression vector after polymerase chain reaction (PCR) amplification from L. monocytogenes DNA. The oligonucleotides used for priming were 5′-CCCGGGATCCACCATGAAAAAAATAATGCTAG-3′ and 5′-GGATCCGGATCCTTATTCGATTGGATTATC-3′ which introduced flanking _Bam_HI sites. Electroporation of _Pvu_I linearized plasmid containing the insert into P815 cells and selection of G418 resistant clones was performed as described. P815 cells transfected with the vector without the insert were not lysed by the LmT CTL line (not shown).
FIG. 4
Exact sequence determination of the H-2Kd-restricted LLO peptide. a, Eleven nonamer peptides that conform to the H-2Kd-binding motif were found in the LLO amino-acid sequence and were synthesized on resin with the F-moc multiple peptide synthesis method (Cambridge Research Biochemicals). The amino acids are numbered according to the sequence of LLO. b, All 11 peptides were used with P815 target cells and CTL assays were performed for 3 h with B9 CTL at an effector to target ratio of 20:1. LLO 91–99 sensitized targets (●) whereas LLO 196–204 (○), LLO 211–219 (△) and all other eight peptides (not shown) did not. c, HPLC _A_220 profile of 2 μg synthetic LLO 91–99 (—) on a shallow acetonitrile gradient (broken line), d, CTL assays of shallow gradient HPLC fractions of 2 μg LLO 91–99 (each fraction diluted 10−6 for assay) (○), mock HPLC fractionation to assure that peptide contamination of the HPLC system had not occurred (△), and a TFA extract of a spleen from an _L. monocytogenes_-infected BALB/c mouse (■). B9 effectors were used to detect targeting activity on 51Cr-labelled P815 cells at a ratio of 10:1.
Comment in
- Synthetic vaccines. Peptides of fortune.
Janeway C. Janeway C. Nature. 1991 Oct 31;353(6347):792. doi: 10.1038/353792a0. Nature. 1991. PMID: 1944552 No abstract available.
Similar articles
- CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo.
Harty JT, Bevan MJ. Harty JT, et al. J Exp Med. 1992 Jun 1;175(6):1531-8. doi: 10.1084/jem.175.6.1531. J Exp Med. 1992. PMID: 1375265 Free PMC article. - Two Listeria monocytogenes CTL epitopes are processed from the same antigen with different efficiencies.
Sijts AJ, Neisig A, Neefjes J, Pamer EG. Sijts AJ, et al. J Immunol. 1996 Jan 15;156(2):683-92. J Immunol. 1996. PMID: 8543821 - Cytotoxic-T-lymphocyte responses to epitopes of listeriolysin O and p60 following infection with Listeria monocytogenes.
Bouwer HG, Hinrichs DJ. Bouwer HG, et al. Infect Immun. 1996 Jul;64(7):2515-22. doi: 10.1128/iai.64.7.2515-2522.1996. Infect Immun. 1996. PMID: 8698474 Free PMC article. - MHC class I restricted T cell responses to Listeria monocytogenes, an intracellular bacterial pathogen.
Finelli A, Kerksiek KM, Allen SE, Marshall N, Mercado R, Pilip I, Busch DH, Pamer EG. Finelli A, et al. Immunol Res. 1999;19(2-3):211-23. doi: 10.1007/BF02786489. Immunol Res. 1999. PMID: 10493175 Review. - MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses.
Pamer EG, Sijts AJ, Villanueva MS, Busch DH, Vijh S. Pamer EG, et al. Immunol Rev. 1997 Aug;158:129-36. doi: 10.1111/j.1600-065x.1997.tb00999.x. Immunol Rev. 1997. PMID: 9314081 Review.
Cited by
- A strategy for making synthetic peptide vaccines.
Ogasawara K, Naruse H, Itoh Y, Gotohda T, Arikawa J, Kida H, Good RA, Onoé K. Ogasawara K, et al. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8995-9. doi: 10.1073/pnas.89.19.8995. Proc Natl Acad Sci U S A. 1992. PMID: 1409595 Free PMC article. - Superior protective immunity against murine listeriosis by combined vaccination with CpG DNA and recombinant Salmonella enterica serovar typhimurium.
Berchtold C, Panthel K, Jellbauer S, Köhn B, Roider E, Partilla M, Heesemann J, Endres S, Bourquin C, Rüssmann H. Berchtold C, et al. Infect Immun. 2009 Dec;77(12):5501-8. doi: 10.1128/IAI.00700-09. Epub 2009 Sep 21. Infect Immun. 2009. PMID: 19797070 Free PMC article. - MHC ligands and peptide motifs: first listing.
Rammensee HG, Friede T, Stevanoviíc S. Rammensee HG, et al. Immunogenetics. 1995;41(4):178-228. doi: 10.1007/BF00172063. Immunogenetics. 1995. PMID: 7890324 Review. No abstract available. - Recombinant Listeria monocytogenes vaccination eliminates papillomavirus-induced tumors and prevents papilloma formation from viral DNA.
Jensen ER, Selvakumar R, Shen H, Ahmed R, Wettstein FO, Miller JF. Jensen ER, et al. J Virol. 1997 Nov;71(11):8467-74. doi: 10.1128/JVI.71.11.8467-8474.1997. J Virol. 1997. PMID: 9343203 Free PMC article. - HLA-A2-restricted cytotoxic T lymphocyte epitopes from human heparanase as novel targets for broad-spectrum tumor immunotherapy.
Chen T, Tang XD, Wan Y, Chen L, Yu ST, Xiong Z, Fang DC, Liang GP, Yang SM. Chen T, et al. Neoplasia. 2008 Sep;10(9):977-86. doi: 10.1593/neo.08576. Neoplasia. 2008. PMID: 18714399 Free PMC article.
References
- Marget W, Seeliger HPR. Infection. 1988;16:175–177. - PubMed
- Bishop DK, Hinrichs DJ. J Immun. 1987;139:2005–2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI019335/AI/NIAID NIH HHS/United States
- R01 AI080619/AI/NIAID NIH HHS/United States
- R01 AI039031-11/AI/NIAID NIH HHS/United States
- R01 AI019335-19/AI/NIAID NIH HHS/United States
- R01 AI039031/AI/NIAID NIH HHS/United States
- R01 AI046653/AI/NIAID NIH HHS/United States
- R01 AI046653-05/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials