Alternative vascularization mechanisms in cancer: Pathology and therapeutic implications - PubMed (original) (raw)
Review
Alternative vascularization mechanisms in cancer: Pathology and therapeutic implications
Balázs Döme et al. Am J Pathol. 2007 Jan.
Abstract
Although cancer cells are not generally controlled by normal regulatory mechanisms, tumor growth is highly dependent on the supply of oxygen, nutrients, and host-derived regulators. It is now established that tumor vasculature is not necessarily derived from endothelial cell sprouting; instead, cancer tissue can acquire its vasculature by co-option of pre-existing vessels, intussusceptive microvascular growth, postnatal vasculogenesis, glomeruloid angiogenesis, or vasculogenic mimicry. The best-known molecular pathway driving tumor vascularization is the hypoxia-adaptation mechanism. However, a broad and diverse spectrum of genetic aberrations is associated with the development of the "angiogenic phenotype." Based on this knowledge, novel forms of antivascular modalities have been developed in the past decade. When applying these targeted therapies, the stage of tumor progression, the type of vascularization of the given cancer tissue, and the molecular machinery behind the vascularization process all need to be considered. A further challenge is finding the most appropriate combinations of antivascular therapies and standard radio- and chemotherapies. This review intends to integrate our recent knowledge in this field into a rational strategy that could be the basis for developing effective clinical modalities using antivascular therapy for cancer.
Figures
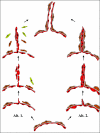
Figure 1
Endothelial sprouting. Schematic representation of the EC sprouting models suggested by Ausprunk and Folkman (Alt. 11) and by Paku and Paweletz (Alt. 22). Red cells represent endothelial cells; brown cells are pericytes. Yellow cells are mural cells of other origin (fibroblasts or bone marrow-derived cells). See Vascularization Mechanisms in Cancer for details.
Figure 2
Examples for vessel co-option. A–D: Pushing-type angiogenesis in liver metastases of colorectal cancer. A: Cross-section of a compressed invagination. SMA-expressing cells (blue fluorescence) facing the tumor tissue, hepatocytes are crowded in the middle of the invagination (pan-cytokeratin, green fluorescence). Continuous CD31 staining (red fluorescence), representing fused sinusoids (arrows), is visible in contact with the SMA-positive cells. Note the paucity of sinusoids between the hepatocytes. B: Laminin (blue fluorescence) co-localizes with α6 integrin within the columns. The column tightly packed with SMA-positive cells (red fluorescence). C: α6 integrin (green fluorescence) is present at the periphery of the column and around the central vessel. D: Schematic representation of the development of vasculature in pushing-type liver metastases. For better visibility of the vessels, hepatocytes are depicted only in the upper part of the drawings. At the early stage of the tumor development, the tumor faces normal liver architecture. As the compression of the tumor grows, the hepatocytes “step back,” and fusion of the sinusoids takes place. The fused vessel, together with the newly synthesized connective tissue, is incorporated into the tumor. The pressure of the tumor results in the separation of the vessel from the liver parenchyma. The vessel in the direction of the axis of the column remains connected to the sinusoidal system of the liver. Column formation is finished by the back-to-back fusion of the basement membranes of the tumor bulges. Green, tumor; brown, hepatocytes; red, sinusoids and central vessel.
Figure 3
Intussusceptive microvascular growth. Schematic representation of intussusceptive microvessel growth. The first step of the process is the development of the transluminal endothelial bridge. This is followed by the reorganization of the endothelial lining, a process that is largely unknown. The division of the vessel is completed by the development of a connective tissue pillar through the vessel lumen. Red cells are endothelial cells; brown cells are pericytes. Gray, basement membrane.
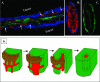
Figure 4
Glomeruloid angiogenesis. A: Experimental brain metastases stained for laminin (green fluorescence) and CD31 (blue fluorescence), 28 days following intracarotid inoculation of the A2058 human melanoma cell line. Glomeruloid bodies are connected to each other by a capillary that is very small in diameter (arrows). The outlines of the metastases are clearly visible because of the strong laminin positivity of the tumor cells (arrowheads). B: Schematic representation of glomeruloid body formation. Following extravasation, the tumor cells (green) adhere firmly to the abluminal surface of the capillary basement membrane (gray). In the first step, because of the contractile force of the tumor cell a loop develops on the capillary. Proliferating tumor cells pull the capillary inward, resulting in the development of further loops and reduction of the diameter of the capillary segment lying outside the glomeruloid body. The last drawing shows the cross-section of a fully developed glomeruloid body built by ECs (red), pericytes (brown), and tumor cells (green). Extreme large cytoplasmic projections of the tumor cells adhere to different segments of the capillary.
Figure 5
Endothelial progenitor cells. Schematic representation of postnatal vasculogenesis. The term “EPC” encompasses a group of cells existing in a variety of stages ranging from common hemangioblasts to fully differentiated ECs. Although their putative precursors and the exact differentiation lineage of EPCs remain to be determined, to date it is widely accepted that early EPCs (localized in the bone marrow or immediately after migration into the circulation) are AC133+/CD34+/VEGFR-2+ cells, whereas circulating EPCs are positive for CD34 and VEGFR-2, lose AC133, and begin to express cell surface markers typical of mature ECs such as CD31, VE-cadherin, and von Willebrand Factor (vWF).
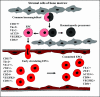
Figure 6
Vasculogenic mimicry. This diagram represents the current interpretation of data generated from several studies involving the use of tracers and perfusion analyses of mice containing aggressive melanoma cells (green) during tumor development. The endothelial-lined vasculature is closely apposed to the tumor cell-formed fluid conducting meshwork, and hypothetically, it is presumed that as the tumor remodels, the vasculature becomes leaky, resulting in the extravascular conduction of plasma. There is also evidence of a physiological connection between the endothelial-lined vasculature and the extravascular melanoma meshwork.
References
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. - PubMed
- Paku S, Paweletz N. First steps of tumor-related angiogenesis. Lab Invest. 1991;65:334–346. - PubMed
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
- Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous