Hedgehog signaling promotes medulloblastoma survival via Bc/II - PubMed (original) (raw)
Hedgehog signaling promotes medulloblastoma survival via Bc/II
Eli E Bar et al. Am J Pathol. 2007 Jan.
Abstract
Activation of the Hedgehog (Hh) pathway has been identified in several cancers, including medulloblastoma, but the mechanisms by which this pathway affects tumor survival and growth are incompletely understood. We investigated whether Hedgehog might promote survival of medulloblastoma cells via up-regulation of BclII. We found that mRNA levels of the Hedgehog pathway effector Gli1 were significantly associated with BclII expression in medulloblastoma and that Gli1 and BclII are both present in regions of decreased apoptosis in nodular medulloblastoma. Transient overexpression of Gli1 and Gli2 in medulloblastoma cultures induced a BclII transcriptional reporter and increased BclII protein levels, whereas stable overexpression of Gli1 was associated with increased BclII mRNA. The Hedgehog antagonist cyclopamine blocked expression of the Hh pathway targets PTCH1 and Gli1, lowered BclII levels, and increased apoptosis in DAOY and UW228 medulloblastoma cells. Apoptotic induction caused by cyclopamine could be rescued in part by enforced expression of Gli1 or BclII. Hh pathway blockade also sensitized medulloblastoma to the effects of the proapoptotic agent lovastatin. These data demonstrate that BclII is an important mediator of Hh activity in medulloblastoma and suggest new strategies for combined chemotherapeutic regimens.
Figures
Figure 1
Expression of _Bcl_II and the Hh targets Gli1 and Gli2 is increased in the internodular regions of nodular/desmoplastic medulloblastoma. a: A cartoon modeling molecular and cellular factors in nodular medulloblastoma. Cells within medulloblastoma nodules have decreased levels of Gli and _Bcl_II, and either differentiate into neurocytes or undergo programmed cell death. b: _Bcl_II expression is low in nodules (asterisk) and high in internodular regions. c: Medulloblastoma cells invading the cerebellar molecular layer (arrows) are strongly immunopositive for Gli1, whereas nonneoplastic internal granule layer neurons (asterisk) are negative. d and e: Gli1 staining was frequently strong in internodular regions (arrowhead) but weak within nodules (asterisk). f and g: Expression of Gli2 was also higher between (arrows) compared with within (asterisks) nodules. Apoptotic cells with condensed nuclei lacked Gli2 protein (small arrow, inset). h: Cleaved caspase 3 was detected in lymphocyte-like apoptotic cells (small arrows). Original magnifications: ×200 (b, d, f, g); ×100 (c); ×400 (e, h).
Figure 2
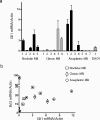
Analysis of Gli and _Bcl_II mRNA expression. a: Gli1 expression was measured by quantitative RT-PCR in RNA extracted from five nodular, six classic, and seven anaplastic snap-frozen medulloblastomas, as well as the DAOY cell line. b: _Bcl_II mRNA levels in these 18 samples are compared with those of Gli1, with each tumor represented by a filled triangle. The subtype of tumors with above-median Gli1 or _Bcl_II expression is further designated using an open square, diamond, or circle.
Figure 3
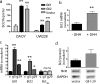
Gli positively up-regulates _Bcl_II expression. a: Transient transfection of constructs encoding Gli1 or Gli2 into DAOY or UW228 cell lines significantly induced transcription of a _Bcl_II promoter-luciferase reporter as compared with empty vector. b: Stimulation of Hh signaling in cerebellar granule cell precursor cultures using SHH ligand results in a significant increase in _Bcl_II mRNA levels measured using quantitative RT-PCR. c: Both Gli1 and _Bcl_II mRNA levels are significantly increased in two DAOY subclones (g5, g29) stably expressing Gli1 compared with a vector-transfected clone (v). d: _Bcl_II protein expression, normalized to GAPDH, is elevated in DAOY cells stably transfected with Gli1. Mean levels from two experiments are shown above representative bands. *P < 0.05, **P < 0.005 for two-sided _t_-tests; ns, not significant.
Figure 4
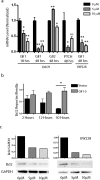
Analysis of Hh targets and _Bcl_II expression after cyclopamine treatment. a: Levels of Gli1 and PTCH1, but not Gli2, are significantly reduced by 5 μmol/L cyclopamine compared with vehicle-treated controls. b: _Bcl_II luciferase reporter assays after 5 μmol/L cyclopamine exposure reveal progressive inhibition of _Bcl_II expression throughout 30 hours. _Bcl_II expression was not inhibited in DAOY cells stably expressing Gli1 (Gli1-5). c: Hedgehog pathway blockade in DAOY and UW228 cells by cyclopamine for 48 hours also inhibited BCL2 protein expression normalized to GAPDH. *P < 0.05, ** P < 0.005 for two-sided _t_-tests; ns, not significant.
Figure 5
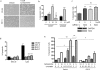
Hh pathway blockade induces apoptosis in medulloblastoma. a: Vector-transfected DAOY cell cultures exposed to 10 μmol/L cyclopamine for 48 hours contained numerous refractile or floating cells. Cell death seemed more limited in cultures treated with vehicle, and in cyclopamine-treated DAOY cells stably expressing Gli1 (Gli1-5). b: Quantification of apoptotic cells after cyclopamine treatment confirmed that it significantly induces apoptosis. Enforced Gli1 expression blocked this increase in cell death in the stable DAOY Gli1-5 subclone. c: DAOY cells were transfected with siRNA targeting _Bcl_II or scrambled control (C) siRNA, and then treated with vehicle or 5 μmol/L cyclopamine. The addition of cyclopamine to cells already depleted of _Bcl_II did not cause significant additional apoptosis. Decreased levels of _Bcl_II protein are documented in a Western blot positioned below the first two bars. d: Two subclones stably transfected with a _Bcl_II-expressing plasmid were not sensitive to apoptotic induction by cyclopamine. e: Combined treatment with cyclopamine and lovastatin acts synergistically to induce apoptosis in medulloblastoma. *P < 0.05, **P < 0.005 for two-sided _t_-tests; ns, not significant.
Similar articles
- Synergism between Hedgehog-GLI and EGFR signaling in Hedgehog-responsive human medulloblastoma cells induces downregulation of canonical Hedgehog-target genes and stabilized expression of GLI1.
Götschel F, Berg D, Gruber W, Bender C, Eberl M, Friedel M, Sonntag J, Rüngeler E, Hache H, Wierling C, Nietfeld W, Lehrach H, Frischauf A, Schwartz-Albiez R, Aberger F, Korf U. Götschel F, et al. PLoS One. 2013 Jun 10;8(6):e65403. doi: 10.1371/journal.pone.0065403. Print 2013. PLoS One. 2013. PMID: 23762360 Free PMC article. - Hedgehog signaling negatively co-regulates BH3-only protein Noxa and TAp73 in TP53-mutated cells.
Meister MT, Boedicker C, Klingebiel T, Fulda S. Meister MT, et al. Cancer Lett. 2018 Aug 10;429:19-28. doi: 10.1016/j.canlet.2018.04.025. Epub 2018 Apr 24. Cancer Lett. 2018. PMID: 29702195 - β-Catenin-Gli1 interaction regulates proliferation and tumor growth in medulloblastoma.
Zinke J, Schneider FT, Harter PN, Thom S, Ziegler N, Toftgård R, Plate KH, Liebner S. Zinke J, et al. Mol Cancer. 2015 Feb 3;14(1):17. doi: 10.1186/s12943-015-0294-4. Mol Cancer. 2015. PMID: 25645196 Free PMC article. - New developments in medulloblastoma treatment: the potential of a cyclopamine-lovastatin combination.
Bar EE, Stearns D. Bar EE, et al. Expert Opin Investig Drugs. 2008 Feb;17(2):185-95. doi: 10.1517/13543784.17.2.185. Expert Opin Investig Drugs. 2008. PMID: 18230052 Review. - Hedgehog beyond medulloblastoma and basal cell carcinoma.
Teglund S, Toftgård R. Teglund S, et al. Biochim Biophys Acta. 2010 Apr;1805(2):181-208. doi: 10.1016/j.bbcan.2010.01.003. Epub 2010 Jan 18. Biochim Biophys Acta. 2010. PMID: 20085802 Review.
Cited by
- Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma.
Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Bar EE, et al. Stem Cells. 2007 Oct;25(10):2524-33. doi: 10.1634/stemcells.2007-0166. Epub 2007 Jul 12. Stem Cells. 2007. PMID: 17628016 Free PMC article. - Stem cells in cancer initiation and progression.
Bajaj J, Diaz E, Reya T. Bajaj J, et al. J Cell Biol. 2020 Jan 6;219(1):e201911053. doi: 10.1083/jcb.201911053. J Cell Biol. 2020. PMID: 31874116 Free PMC article. Review. - Skp2 modulates proliferation, senescence and tumorigenesis of glioma.
Wu J, Su HK, Yu ZH, Xi SY, Guo CC, Hu ZY, Qu Y, Cai HP, Zhao YY, Zhao HF, Chen FR, Huang YF, To ST, Feng BH, Sai K, Chen ZP, Wang J. Wu J, et al. Cancer Cell Int. 2020 Mar 6;20:71. doi: 10.1186/s12935-020-1144-z. eCollection 2020. Cancer Cell Int. 2020. PMID: 32165861 Free PMC article. - Structure-activity relationships and cancer-cell selective toxicity of novel inhibitors of glioma-associated oncogene homologue 1 (Gli1) mediated transcription.
Mahindroo N, Connelly MC, Punchihewa C, Kimura H, Smeltzer MP, Wu S, Fujii N. Mahindroo N, et al. J Med Chem. 2009 Jul 23;52(14):4277-87. doi: 10.1021/jm900106f. J Med Chem. 2009. PMID: 19545120 Free PMC article. - Zinc and zinc-containing biomolecules in childhood brain tumors.
Hrabeta J, Eckschlager T, Stiborova M, Heger Z, Krizkova S, Adam V. Hrabeta J, et al. J Mol Med (Berl). 2016 Nov;94(11):1199-1215. doi: 10.1007/s00109-016-1454-8. Epub 2016 Sep 16. J Mol Med (Berl). 2016. PMID: 27638340 Review.
References
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, Beachy PA. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. - PubMed
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. - PubMed
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. - PubMed
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources