Highly efficient concerted evolution in the ribosomal DNA repeats: total rDNA repeat variation revealed by whole-genome shotgun sequence data - PubMed (original) (raw)
Comparative Study
Highly efficient concerted evolution in the ribosomal DNA repeats: total rDNA repeat variation revealed by whole-genome shotgun sequence data
Austen R D Ganley et al. Genome Res. 2007 Feb.
Abstract
Repeat families within genomes are often maintained with similar sequences. Traditionally, this has been explained by concerted evolution, where repeats in an array evolve "in concert" with the same sequence via continual turnover of repeats by recombination. Another form of evolution, birth-and-death evolution, can also explain this pattern, although in this case selection is the critical force maintaining the repeats. The level of intragenomic variation is the key difference between these two forms of evolution. The prohibitive size and repetitive nature of large repeat arrays have made determination of the absolute level of intragenomic repeat variability difficult, thus there is little evidence to support concerted evolution over birth-and-death evolution for many large repeat arrays. Here we use whole-genome shotgun sequence data from the genome projects of five fungal species to reveal absolute levels of sequence variation within the ribosomal RNA gene repeats (rDNA). The level of sequence variation is remarkably low. Furthermore, the polymorphisms that are detected are not functionally constrained and seem to exist beneath the level of selection. These results suggest the rDNA is evolving via concerted evolution. Comparisons with a repeat array undergoing birth-and-death evolution provide a clear contrast in the level of repeat array variation between these two forms of evolution, confirming that the rDNA indeed does evolve via concerted evolution. These low levels of intra-genomic variation are consistent with a model of concerted evolution in which homogenization is very rapid and efficiently maintains highly similar repeat arrays.
Figures
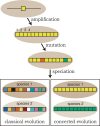
Figure 1.
Classical evolution vs. concerted evolution. Repeats (individual boxes) in a repeat array are initially formed by a gene amplification event. The repeats accumulate mutations (alternatively colored boxes) through time. Under classical evolution these mutations persist and therefore, after speciation events, the orthologous relationships of the repeats remain (e.g., repeat #1 from species 1 will resemble repeat #1 from species 2 more closely than the other repeats in species 1; indicated schematically by different shades of the same color). However, under concerted evolution, homogenization continuously sweeps one repeat variant (at random) to fixation within the array. Therefore, the repeats within a genome are all expected to be similar, but differ in sequence from the repeats in a closely related species. Birth-and-death evolution is a more complex form of classical evolution mixed with some aspects of concerted evolution, and is not depicted.
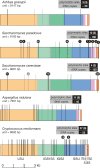
Figure 2.
Positions of the polymorphisms in the rDNA unit. Polymorphic sites for each species are plotted onto a map of a single rDNA unit. rDNA features are color coded as indicated beneath the bottom rDNA unit. High-confidence polymorphisms are shown as black lines, low-confidence polymorphisms as gray lines, and the total numbers are boxed (low-confidence polymorphisms in parentheses). Although the polymorphisms are shown in a single repeat, in reality they are likely to be scattered throughout the array. Polymorphisms present in more than one sequence read are indicated by black balls above the line, with the number of reads shown in the ball. In many cases these are likely to result from the coverage level of the whole-genome shotgun sequence data. The rDNA unit length and copy number (from Fig. 3) are also indicated (S. paradoxus is the diploid copy number). Diagram is to scale.
Figure 3.
Determination of rDNA array sizes by pulsed field gel electrophoresis. (A) Ethidium bromide-stained gels showing the sizes of the rDNA arrays from A. nidulans, S. paradoxus, and C. neoformans after digestion of chromosomal plugs with HinDIII, BamHI, and AgeI, respectively. (B) Southern blot of the gels from A probed with a conserved region of the LSU from S. cerevisiae to confirm the rDNA bands. Array sizes (kilobase) are indicated, as are rDNA copy numbers (in parentheses), and these were calculated using S. cerevisiae chromosomes as size markers (M).
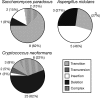
Figure 4.
Mutational profile of the rDNA polymorphisms. The proportion of each of the five classes of mutation (listed in the boxed legend) that form the high-confidence polymorphisms are graphed for S. paradoxus, A. nidulans, and C. neoformans. Absolute numbers and percentages are given for each class. “Complex” mutations are defined as those involving more than 3 bp. For the full list of polymorphisms, see Supplemental Table 1.
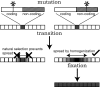
Figure 5.
Three phases of repeat homogenization under a rapid homogenization model. First, a mutation occurs at either a selectively constrained (e.g., a coding part of the repeat), or a nonselectively constrained (e.g., a noncoding part of the repeat) site in a single unit from the stylized array. In the transition phase, only the unit with the nonselectively constrained mutation can increase to high copy number by homogenization. This mutation is able to sweep to fixation in the array. Thus, only mutations tolerated by selection can spread throughout the array, explaining why within the same repeat some regions are highly polymorphic while others are highly conserved, even though the entire repeat unit is subject to the identical homogenization process. See text for details.
Similar articles
- Monitoring the rate and dynamics of concerted evolution in the ribosomal DNA repeats of Saccharomyces cerevisiae using experimental evolution.
Ganley AR, Kobayashi T. Ganley AR, et al. Mol Biol Evol. 2011 Oct;28(10):2883-91. doi: 10.1093/molbev/msr117. Epub 2011 May 4. Mol Biol Evol. 2011. PMID: 21546356 - Contrasting Patterns of rDNA Homogenization within the Zygosaccharomyces rouxii Species Complex.
Chand Dakal T, Giudici P, Solieri L. Chand Dakal T, et al. PLoS One. 2016 Aug 8;11(8):e0160744. doi: 10.1371/journal.pone.0160744. eCollection 2016. PLoS One. 2016. PMID: 27501051 Free PMC article. - Repetitive sequence variation and dynamics in the ribosomal DNA array of Saccharomyces cerevisiae as revealed by whole-genome resequencing.
James SA, O'Kelly MJ, Carter DM, Davey RP, van Oudenaarden A, Roberts IN. James SA, et al. Genome Res. 2009 Apr;19(4):626-35. doi: 10.1101/gr.084517.108. Epub 2009 Jan 13. Genome Res. 2009. PMID: 19141593 Free PMC article. - Intragenomic rDNA variation - the product of concerted evolution, mutation, or something in between?
Wang W, Zhang X, Garcia S, Leitch AR, Kovařík A. Wang W, et al. Heredity (Edinb). 2023 Sep;131(3):179-188. doi: 10.1038/s41437-023-00634-5. Epub 2023 Jul 4. Heredity (Edinb). 2023. PMID: 37402824 Free PMC article. Review. - To Repeat or Not to Repeat: Repetitive Sequences Regulate Genome Stability in Candida albicans.
Dunn MJ, Anderson MZ. Dunn MJ, et al. Genes (Basel). 2019 Oct 30;10(11):866. doi: 10.3390/genes10110866. Genes (Basel). 2019. PMID: 31671659 Free PMC article. Review.
Cited by
- Variation of natural selection in the Amoebozoa reveals heterogeneity across the phylogeny and adaptive evolution in diverse lineages.
Wang F, Tekle YI. Wang F, et al. Front Ecol Evol. 2022;10:851816. doi: 10.3389/fevo.2022.851816. Epub 2022 Aug 4. Front Ecol Evol. 2022. PMID: 36874909 Free PMC article. - Species recognition and clinical relevance of the zygomycetous genus Lichtheimia (syn. Absidia pro parte, Mycocladus).
Alastruey-Izquierdo A, Hoffmann K, de Hoog GS, Rodriguez-Tudela JL, Voigt K, Bibashi E, Walther G. Alastruey-Izquierdo A, et al. J Clin Microbiol. 2010 Jun;48(6):2154-70. doi: 10.1128/JCM.01744-09. Epub 2010 Mar 31. J Clin Microbiol. 2010. PMID: 20357218 Free PMC article. - Intragenic homogenization and multiple copies of prey-wrapping silk genes in Argiope garden spiders.
Chaw RC, Zhao Y, Wei J, Ayoub NA, Allen R, Atrushi K, Hayashi CY. Chaw RC, et al. BMC Evol Biol. 2014 Feb 20;14:31. doi: 10.1186/1471-2148-14-31. BMC Evol Biol. 2014. PMID: 24552485 Free PMC article. - Comparative Studies on the Polymorphism and Copy Number Variation of mtSSU rDNA in Ciliates (Protista, Ciliophora): Implications for Phylogenetic, Environmental, and Ecological Research.
Wang Y, Jiang Y, Liu Y, Li Y, Katz LA, Gao F, Yan Y. Wang Y, et al. Microorganisms. 2020 Feb 25;8(3):316. doi: 10.3390/microorganisms8030316. Microorganisms. 2020. PMID: 32106521 Free PMC article. - Are ribosomal DNA clusters rearrangement hotspots?: a case study in the genus Mus (Rodentia, Muridae).
Cazaux B, Catalan J, Veyrunes F, Douzery EJ, Britton-Davidian J. Cazaux B, et al. BMC Evol Biol. 2011 May 13;11:124. doi: 10.1186/1471-2148-11-124. BMC Evol Biol. 2011. PMID: 21569527 Free PMC article.
References
- Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J., Zhang J., Zhang Z., Miller W., Lipman D.J., Zhang Z., Miller W., Lipman D.J., Miller W., Lipman D.J., Lipman D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. - PMC - PubMed
- Andersson D.I., Slechta E.S., Roth J.R., Slechta E.S., Roth J.R., Roth J.R. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science. 1998;282:1133–1135. - PubMed
- Ben Ali A., Wuyts J., De Wachter R., Meyer A., de Van Peer Y., Wuyts J., De Wachter R., Meyer A., de Van Peer Y., De Wachter R., Meyer A., de Van Peer Y., Meyer A., de Van Peer Y., de Van Peer Y. Construction of a variability map for eukaryotic large subunit ribosomal RNA. Nucleic Acids Res. 1999;27:2825–2831. - PMC - PubMed
- Berbee M.L., Taylor J.W., Taylor J.W. Fungal molecular evolution: Gene trees and geologic time. In: McLaughlin D.J., McLaughlin E., Lemke P.A., McLaughlin E., Lemke P.A., Lemke P.A., editors. The Mycota. Springer; Berlin: 2001. pp. 229–246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases