Enhanced mast cell activation in mice deficient in the A2b adenosine receptor - PubMed (original) (raw)
Enhanced mast cell activation in mice deficient in the A2b adenosine receptor
Xiaoyang Hua et al. J Exp Med. 2007.
Erratum in
- J Exp Med. 2007 Apr 16;204(4):963
Abstract
Antigen-mediated cross-linking of IgE bound to mast cells via the high affinity receptor for IgE triggers a signaling cascade that results in the release of intracellular calcium stores, followed by an influx of extracellular calcium. The collective increase in intracellular calcium is critical to the release of the granular contents of the mast cell, which include the mediators of acute anaphylaxis. We show that the sensitivity of the mast cell to antigen-mediated degranulation through this pathway can be dramatically influenced by the A2b adenosine receptor. Loss of this Gs-coupled receptor on mouse bone marrow-derived mast cells results in decreased basal levels of cyclic AMP and an excessive influx of extracellular calcium through store-operated calcium channels following antigen activation. Mice lacking the A2b receptor display increased sensitivity to IgE-mediated anaphylaxis. Collectively, these findings show that the A2b adenosine receptor functions as a critical regulator of signaling pathways within the mast cell, which act in concert to limit the magnitude of mast cell responsiveness when antigen is encountered.
Figures
Figure 1.
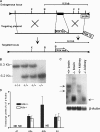
Targeted disruption of the mouse A2b gene. (A) The targeted disruption strategy consists of a targeting plasmid and genomic organization of the endogenous locus and targeted locus. The targeting plasmid is designed to disrupt the A2b gene by homologous recombination. The closed box represents exon 2 of the A2b gene, and the phosphoglycerate kinase–thymidine kinase (PGK-TK) and PGK-Neo selection cassettes are indicated by empty boxes. Restriction sites are abbreviated as follows: X, Xba I; B, BamH I; and P, Psi I. The location of the 3′ external probe used to detect homologous recombination events for the Southern blot analysis is indicated. (B) Southern blot analysis of _BamH I_–digested mouse genomic DNA from tail biopsies. Blots were hybridized with the radiolabeled 1-kb probe outside of the targeted region of the A2b gene, resulting in an 8.3-kb band for the endogenous locus and a band shift to 6.2 kb for the targeted locus. (C) Northern blot analysis of A2b mRNA in A2b−/− and A2b+/+ mice. Total RNA extracts from the brain and kidney of A2b−/− and A2b+/+ mice were isolated and hybridized with a [32P]UTP-labeled cDNA probe (top) and β-actin probe (bottom) as loading controls. The arrows on the left indicate the two transcripts from the A2b+/+ mice, and the arrow on the right shows the presence of the truncated transcript in the targeted locus. 20 μg of total RNA was loaded for each lane. (D) Quantitative RT-PCR analysis of mRNA level of adenosine receptors in A2b+/+ versus A2b−/− BMMCs. Data are expressed as the mean transcript level ± SEM from three sets of A2b−/− and three sets of A2b+/+ cells. ND, no transcripts detected.
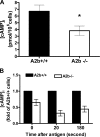
Figure 2.
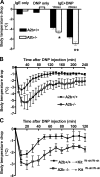
PSA in A2b+/+, A2b−/−, and reconstituted C57BL/6 kitW-sh/W-sh mice. Mice were intravenously loaded with 20 μg (A, n = 14 per group; and C, n = 5–6 per group) or 2 μg (B, n = 6 per group) of anti-DNP IgE and challenged with DNP-HSA 24 h later. Data represent the mean body temperature drop over time after DNP-HSA challenge ± SEM. P < 0.05 by repeated measurement analysis of variance (ANOVA; A– C). *, P < 0.05; and **, P < 0.01 versus A2b+/+ mice by t test.
Figure 3.
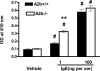
PCA in A2b+/+ and A2b−/− mice. Mice were intradermally injected in the right ears with anti-DNP IgE in 20 μl PBS and in the left ears with aliquot vehicle. DNP-HSA/Evans blue solution was intravenously administered 24 h later, and Evans blue extravasation into the ears was measured. Data represent the mean OD ± SEM (n = 10 mice per group). #, P < 0.0001 versus vehicle-treated ears by t test; **, P = 7.36 × 10−6 versus A2b+/+ ears by t test.
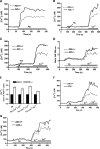
Figure 4.
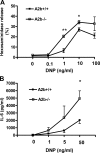
Antigen-induced degranulation and cytokine production from A2b+/+ and A2b−/− mast cells. (A) Degranulation of BMMCs was assessed by measuring hexosamindase release from cells incubated with anti-DNP IgE after stimulation with increasing doses of DNP-HSA antigen. Data are from three different experiments using three different pairs of cells and are expressed as the mean percent hexosamindase release ± SEM. P < 0.001 by ANOVA. *, P < 0.05; and **, P < 0.005 versus A2b+/+ cells by t test. (B) Cytokine production by BMMCs was evaluated by measuring IL-6 release 7 h after antigen stimulation. Cells were incubated with anti-DNP IgE and stimulated with 1, 5, or 50 ng/ml DNP-HSA antigen or vehicle, and cytokine levels were determined by ELISA. Data are from four different experiments using three different pairs of cells and are expressed as the mean IL-6 protein level ± SEM. P < 0.05 by ANOVA. *, P < 0.05 versus A2b+/+ cells by t test.
Figure 5.
Intracellular cAMP levels in A2b+/+ and A2b−/− mast cells. (A) Basal cAMP level in BMMCs from A2b+/+ and A2b−/− mice was determined by ELISA. Data are from three separate experiments using three sets of cells and are expressed as the mean ± SEM. *, P = 0.03 versus A2b+/+ cells by t test. (B) cAMP level in both A2b−/− and A2b+/+ mast cells at the indicated times after DNP challenge. Data are expressed as fold cAMP level relative to A2b+/+ cells, ± SEM.
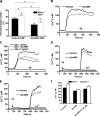
Figure 6.
Calcium influx in A2b+/+ and A2b−/− mast cells. (A–C) Calcium influx in A2b+/+ and A2b−/− BMMCs (anti-DNP IgE preloaded) in calcium (A) or calcium-free (B and C) buffer were treated with 20 ng/ml DNP-HSA (A and B) or 1 μM TG (C) and, cytosolic-free calcium was measured as shown. When cells were suspended in calcium-free buffer, extracellular calcium was repleted to final a concentration of 1 mM after DNP-HSA or TG challenge. (D) Increased Ca2+ surrogate Sr2+ influx in A2b−/− cells. A2b+/+ or A2b−/− BMMCs in calcium-free buffer were challenged with 1 μM TG, and Sr2+ was added to a final concentration of 1 mM. Sr2+ influx was recorded as shown. (E) Mean fold increase of intracellular Ca2+ or Sr2+ concentrations, ± SEM, in A2b−/− cells compared with A2b+/+ cells after Fc_ɛ_RI-mediated activation. (F and G) Increased Ca2+ surrogate Sr2+ influx in A2b−/− cells. A2b−/− and A2b+/+ BMMCs (anti-DNP IgE preloaded) in calcium-free buffer were triggered by DNP-HSA (F) or TG (G), and bivalent cations were recovered by successive addition of 1 mM Sr2+ and 1 mM Ca2+ as shown. Representative results from at least three independent experiments and two sets of cells are shown. Ag, antigen.
Figure 7.
Inhibitory effect of cAMP analogue on hexosaminidase release and SOCCs on mast cells. (A) Inhibitory effect of cAMP analogue on antigen-induced degranulation of both A2b+/+ and A2b−/− mast cells. Anti-DNP IgE preloaded A2b−/− and A2b+/+ BMMCs were treated with 3 mM dbcAMP for 30min before the challenge with 10 ng/ml DNP, and the effect of dbcAMP on antigen-induced degranulation of A2b−/− and A2b+/+ BMMCs was measures as shown. Data represent mean hexosaminidase release, ± SEM, from four separated experiments using three different sets of cells. *, P < 0.05 by t test. (B–E) Inhibitory effect of cAMP analogue on antigen- (B–D) or TG-induced (E) calcium influx in both A2b+/+ and A2b−/− mast cells. Both A2b−/− and A2b+/+ BMMCs in calcium (B and C) or calcium-free (D and E) buffer were treated with dbcAMP buffer were treated with dbcAMP and DNP-HSA (B–D), or TG-induced (E) calcium influx was recorded. When cells were suspended in calcium-free buffer (D and E), extracellular calcium was restored 10 min after the addition of antigen (D) or TG (E). Representative results from at least three independent experiments and two sets of cells are shown. (F) Anti-DNP IgE preloaded A2b−/− and A2b+/+ BMMCs were treated with 50 nM of PKA inhibitor KT5720 for 30 min, and the dbcAMP effect on antigen-induced calcium influx was reevaluated as shown. Data represent mean [Ca2+]i, ± SEM, from three independent experiments and two sets of cells. Ag, antigen.
Similar articles
- Adenosine Signaling in Mast Cells and Allergic Diseases.
Garcia-Garcia L, Olle L, Martin M, Roca-Ferrer J, Muñoz-Cano R. Garcia-Garcia L, et al. Int J Mol Sci. 2021 May 14;22(10):5203. doi: 10.3390/ijms22105203. Int J Mol Sci. 2021. PMID: 34068999 Free PMC article. Review. - Deletion of Orai2 augments endogenous CRAC currents and degranulation in mast cells leading to enhanced anaphylaxis.
Tsvilovskyy V, Solís-López A, Schumacher D, Medert R, Roers A, Kriebs U, Freichel M. Tsvilovskyy V, et al. Cell Calcium. 2018 May;71:24-33. doi: 10.1016/j.ceca.2017.11.004. Epub 2017 Nov 27. Cell Calcium. 2018. PMID: 29604961 - Tespa1 negatively regulates FcεRI-mediated signaling and the mast cell-mediated allergic response.
Wang D, Zheng M, Qiu Y, Guo C, Ji J, Lei L, Zhang X, Liang J, Lou J, Huang W, Dong B, Wu S, Wang J, Ke Y, Cao X, Zhou YT, Lu L. Wang D, et al. J Exp Med. 2014 Dec 15;211(13):2635-49. doi: 10.1084/jem.20140470. Epub 2014 Nov 24. J Exp Med. 2014. PMID: 25422497 Free PMC article. - Gs-coupled adenosine receptors differentially limit antigen-induced mast cell activation.
Hua X, Chason KD, Jania C, Acosta T, Ledent C, Tilley SL. Hua X, et al. J Pharmacol Exp Ther. 2013 Feb;344(2):426-35. doi: 10.1124/jpet.112.198978. Epub 2012 Nov 13. J Pharmacol Exp Ther. 2013. PMID: 23149337 Free PMC article. - Mast Cells and Anaphylaxis.
Lieberman P, Garvey LH. Lieberman P, et al. Curr Allergy Asthma Rep. 2016 Mar;16(3):20. doi: 10.1007/s11882-016-0598-5. Curr Allergy Asthma Rep. 2016. PMID: 26857018 Review.
Cited by
- Adenosine Signaling in Mast Cells and Allergic Diseases.
Garcia-Garcia L, Olle L, Martin M, Roca-Ferrer J, Muñoz-Cano R. Garcia-Garcia L, et al. Int J Mol Sci. 2021 May 14;22(10):5203. doi: 10.3390/ijms22105203. Int J Mol Sci. 2021. PMID: 34068999 Free PMC article. Review. - The Many Faces of the A2b Adenosine Receptor in Cardiovascular and Metabolic Diseases.
Eisenstein A, Patterson S, Ravid K. Eisenstein A, et al. J Cell Physiol. 2015 Dec;230(12):2891-7. doi: 10.1002/jcp.25043. J Cell Physiol. 2015. PMID: 25975415 Free PMC article. Review. - Adenosine deaminase inhibition prevents Clostridium difficile toxin A-induced enteritis in mice.
de Araújo Junqueira AF, Dias AA, Vale ML, Spilborghs GM, Bossa AS, Lima BB, Carvalho AF, Guerrant RL, Ribeiro RA, Brito GA. de Araújo Junqueira AF, et al. Infect Immun. 2011 Feb;79(2):653-62. doi: 10.1128/IAI.01159-10. Epub 2010 Nov 29. Infect Immun. 2011. PMID: 21115723 Free PMC article. - Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells.
Koda K, Salazar-Rodriguez M, Corti F, Chan NY, Estephan R, Silver RB, Mochly-Rosen D, Levi R. Koda K, et al. Circulation. 2010 Aug 24;122(8):771-81. doi: 10.1161/CIRCULATIONAHA.110.952481. Epub 2010 Aug 9. Circulation. 2010. PMID: 20697027 Free PMC article. - Histamine H4-receptors inhibit mast cell renin release in ischemia/reperfusion via protein kinase C ε-dependent aldehyde dehydrogenase type-2 activation.
Aldi S, Takano K, Tomita K, Koda K, Chan NY, Marino A, Salazar-Rodriguez M, Thurmond RL, Levi R. Aldi S, et al. J Pharmacol Exp Ther. 2014 Jun;349(3):508-17. doi: 10.1124/jpet.114.214122. Epub 2014 Apr 2. J Pharmacol Exp Ther. 2014. PMID: 24696042 Free PMC article.
References
- Galli, S.J., S. Nakae, and M. Tsai. 2005. Mast cells in the development of adaptive immune responses. Nat. Immunol. 6:135–142. - PubMed
- Metcalfe, D.D., D. Baram, and Y.A. Mekori. 1997. Mast cells. Physiol. Rev. 77:1033–1079. - PubMed
- Marquardt, D.L., C.W. Parker, and T.J. Sullivan. 1978. Potentiation of mast cell mediator release by adenosine. J. Immunol. 120:871–878. - PubMed
- Suzuki, H., M. Takei, T. Nakahata, and H. Fukamachi. 1998. Inhibitory effect of adenosine on degranulation of human cultured mast cells upon cross-linking of Fc epsilon RI. Biochem. Biophys. Res. Commun. 242:697–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials