Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta - PubMed (original) (raw)
Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta
Suchita Nadkarni et al. J Exp Med. 2007.
Erratum in
- J Exp Med. 2007 Jan 22;204(1):205
Abstract
The induction of regulatory T (T reg) cells holds considerable potential as a treatment for autoimmune diseases. We have previously shown that CD4+CD25hi T reg cells isolated from patients with active rheumatoid arthritis (RA) have a defect in their ability to suppress proinflammatory cytokine production by CD4+CD25- [corrected] T cells. This defect, however, was overcome after anti-tumor necrosis factor (TNF)-alpha antibody (infliximab) therapy. Here, we demonstrate that infliximab therapy gives rise to a CD4+CD25hiFoxP3+ T reg cell population, which mediates suppression via transforming growth factor (TGF)-beta and interleukin 10, and lacks CD62L expression, thereby distinguishing this T reg cell subset from natural T reg cells present in healthy individuals and patients with active RA. In vitro, infliximab induced the differentiation of CD62L- T reg cells from CD4+CD25- T cells isolated from active RA patients, a process dependent on TGF-beta. In spite of the potent suppressor capacity displayed by this CD62L- T reg cell population, the natural CD62L+ T reg cells remained defective in infliximab-treated patients. These results suggest that anti-TNF-alpha therapy in RA patients generates a newly differentiated population of T reg cells, which compensates for the defective natural T reg cells. Therefore, manipulation of a proinflammatory environment could represent a therapeutic strategy for the induction of T reg cells and the restoration of tolerance.
Figures
Figure 1.
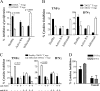
Increased numbers of Foxp3+CD62L−CD4+ T cells found in infliximab-treated RA patients compared with patients with active disease and healthy controls. (A) Representative FACS plots gated on the CD4+ population depicting PBMCs from healthy controls, active RA patients, and infliximab-treated RA patients stained with anti-CD4 and anti-Foxp3. The percentages of Foxp3+ cells in the CD4+ gate for individual RA patients, before and after infliximab, and healthy controls are shown in the chart. (B) Histograms were gated on CD4+CD25high cells (as indicated), and the expression of CD62L is shown. (C) PBMCs from the same groups as in A were stained with anti-CD4, anti-Foxp3, and anti-CD62L. The histograms indicate the expression of CD62L in the CD4+Foxp3+ population. Data from individual patients are shown in the chart. (D) CCR7 and CD45RO expression on the CD4+Foxp3+ T cell population in the different patient groups. (E) CCR7 and CD45RO expression on the CD4+FoxP3+CD62L− population from an RA patient treated with infliximab. Results are representative of six patients or healthy individuals for each group. The dashed line in the FACS plots represents the isotype control for the specific marker examined.
Figure 2.
CD62L− T reg cells from infliximab-treated RA patients are more potent suppressors than their CD62L+ counterparts and mediate their suppressive effects through IL-10 and TGF-β. CD4+CD25−, CD4+CD25hiCD62L+, and CD4+CD25hiCD62L− were FACS (MoFlo) sorted from the PBMCs of healthy controls, active RA patients, and infliximab-treated RA patients. In all experiments, cells were stimulated with 2 μg/ml of soluble anti-CD3/CD28. (A) CD4+CD25− T cells were cocultured with either CD62L+ or CD62L− T reg cells (2:1 ratio shown) for 5 d, with [3H]thymidine added in the last 18 h of culture. Mean triplicate values shown from six patients. (B) CD4+CD25− T cells were cultured alone or cocultured at a 2:1 ratio with either CD62L+ or CD62L− T reg cells for 48 h. Cells were intracellularly stained for TNF-α and IFN-γ. Data shown expressed as mean ± SE of six patients and six healthy controls, and are represented as the percentage inhibition of cytokine production compared with CD4+CD25− T cells alone. The means for percent IFN-γ+/TNF-α+ cells are as follows: healthy CD4+CD25− 3.2/3.4, CD4+CD25−/CD62L+ T reg cells 0.7/0.9, CD4+CD25−/CD62L− T reg cells 1.9/2.1; active RA CD4+CD25− 5.2/13.2, CD4+CD25−/CD62L+ T reg cells 3.6/9.0, CD4+CD25−/CD62L− T reg cells 3.1/7.8; post-infliximab CD4+CD25− 4.2/8.6, CD4+CD25−/CD62L+ T reg cells 3.8/6.3; and CD4+CD25−/CD62L− T reg cells 1.1/2.2. (C) CD4+CD25− T cells from healthy and infliximab-treated RA patients were cultured alone or with CD62L− T reg cells (2:1 ratio) for 48 h with anti-CD3/CD28 alone or in the presence of 2 μg/ml anti–TGF-β1, 0.5 μg/ml anti–IL-10, or anti–TGF-β1 and anti–IL-10 together, and stained for TNF-α and IFN-γ. Data depicted represent mean ± SE of six patients and healthy controls. (D) CD4+CD25− T cells were cocultured, either directly or separated by a transwell membrane, with CD62L− T reg cells from RA patients after infliximab, and stimulated with 2 μg/ml anti-CD3/CD28 for 48 h. Cells were intracellularly stained for TNF-α and IFN-γ, and the results are depicted as the percentage inhibition of cytokine production compared with CD4+CD25− T cells alone. Data represent mean ± SE of four patients.
Figure 3.
The addition of infliximab in vitro to activated RA CD4+CD25− T cells induced a population of FoxP3+ T cells that were functionally suppressive. (A) Purified CD4+CD25− T cells from both healthy controls and active RA patients were stimulated with 2 μg/ml anti-CD3/CD28, ±10 μg/ml infliximab. In some wells, 2 μg/ml anti–TGF-β was added. Cells were cultured for 24 h and then intracellularly stained for FoxP3. Representative FACS plots are shown as well as the pooled data (n = 8 patients and 8 healthy individuals). Percentages shown are of the purified CD4+CD25− fraction. (B) Supernatants were collected from cells cultured in A before staining and were tested for TGF-β production by ELISA. (C) Purified CD4+CD25high T cells from healthy individuals and patients with active RA were incubated with 2 μg/ml anti-CD3/CD28, ±10 μg/ml infliximab, and the percentages of Foxp3+ cells are shown. (D) Histograms depicting CD62L expression in the T cells after culture as in A. Black line, isotype control; shaded area, CD62L. (E) CD4+CD25+ from the cultures in A were isolated, recultured at a 1:2 ratio with freshly isolated autologous CD4+CD25− T cells for an additional 2 d, and stained for TNF-α and IFN-γ. Data are depicted as the percentage of cytokine inhibition (n = 8).
Similar articles
- Increased spontaneous apoptosis of CD4+CD25+ T cells in patients with active rheumatoid arthritis is reduced by infliximab.
Toubi E, Kessel A, Mahmudov Z, Hallas K, Rozenbaum M, Rosner I. Toubi E, et al. Ann N Y Acad Sci. 2005 Jun;1051:506-14. doi: 10.1196/annals.1361.095. Ann N Y Acad Sci. 2005. PMID: 16126991 - CD4(+)CD25 (+) regulatory T cells in human lupus erythematosus.
Kuhn A, Beissert S, Krammer PH. Kuhn A, et al. Arch Dermatol Res. 2009 Jan;301(1):71-81. doi: 10.1007/s00403-008-0891-9. Epub 2008 Nov 5. Arch Dermatol Res. 2009. PMID: 18985367 Review. - Rescuing CD4+CD25+ regulatory T-cell functions in rheumatoid arthritis by cytokine-targeted monoclonal antibody therapy.
Bayry J, Sibéril S, Triebel F, Tough DF, Kaveri SV. Bayry J, et al. Drug Discov Today. 2007 Jul;12(13-14):548-52. doi: 10.1016/j.drudis.2007.05.002. Epub 2007 Jun 27. Drug Discov Today. 2007. PMID: 17631249 Review. - Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to disease activity score-28.
Sempere-Ortells JM, Pérez-García V, Marín-Alberca G, Peris-Pertusa A, Benito JM, Marco FM, Zubcoff JJ, Navarro-Blasco FJ. Sempere-Ortells JM, et al. Autoimmunity. 2009;42(8):636-45. doi: 10.3109/08916930903061491. Autoimmunity. 2009. PMID: 19886735 - TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets.
Pyzik M, Piccirillo CA. Pyzik M, et al. J Leukoc Biol. 2007 Aug;82(2):335-46. doi: 10.1189/jlb.1006644. Epub 2007 May 2. J Leukoc Biol. 2007. PMID: 17475784
Cited by
- Helicobacter pylori induces in-vivo expansion of human regulatory T cells through stimulating interleukin-1β production by dendritic cells.
Mitchell PJ, Afzali B, Fazekasova H, Chen D, Ali N, Powell N, Lord GM, Lechler RI, Lombardi G. Mitchell PJ, et al. Clin Exp Immunol. 2012 Dec;170(3):300-9. doi: 10.1111/j.1365-2249.2012.04659.x. Clin Exp Immunol. 2012. PMID: 23121671 Free PMC article. - T cells out of control--impaired immune regulation in the inflamed joint.
Wehrens EJ, Prakken BJ, van Wijk F. Wehrens EJ, et al. Nat Rev Rheumatol. 2013 Jan;9(1):34-42. doi: 10.1038/nrrheum.2012.149. Nat Rev Rheumatol. 2013. PMID: 23390638 Review. - Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis.
Shen S, Shin JJ, Strle K, McHugh G, Li X, Glickstein LJ, Drouin EE, Steere AC. Shen S, et al. Arthritis Rheum. 2010 Jul;62(7):2127-37. doi: 10.1002/art.27468. Arthritis Rheum. 2010. PMID: 20506317 Free PMC article. - Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis.
Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Flier SN, et al. J Biol Chem. 2010 Jun 25;285(26):20202-12. doi: 10.1074/jbc.M110.102012. Epub 2010 Apr 2. J Biol Chem. 2010. PMID: 20363741 Free PMC article. - Treg cells in autoimmunity: from identification to Treg-based therapies.
Göschl L, Scheinecker C, Bonelli M. Göschl L, et al. Semin Immunopathol. 2019 May;41(3):301-314. doi: 10.1007/s00281-019-00741-8. Epub 2019 Apr 5. Semin Immunopathol. 2019. PMID: 30953162 Review.
References
- Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. - PubMed
- Levings, M.K., and M.G. Roncarolo. 2005. Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr. Top. Microbiol. Immunol. 293:303–326. - PubMed
- Bluestone, J.A., and A.K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253–257. - PubMed
- Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials