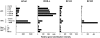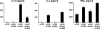Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand - PubMed (original) (raw)
Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand
Tomoki Ito et al. J Exp Med. 2007.
Abstract
Although there is evidence for distinct roles of myeloid dendritic cells (DCs [mDCs]) and plasmacytoid pre-DCs (pDCs) in regulating T cell-mediated adaptive immunity, the concept of functional DC subsets has been questioned because of the lack of a molecular mechanism to explain these differences. In this study, we provide direct evidence that maturing mDCs and pDCs express different sets of molecules for T cell priming. Although both maturing mDCs and pDCs upregulate the expression of CD80 and CD86, only pDCs upregulate the expression of inducible costimulator ligand (ICOS-L) and maintain high expression levels upon differentiation into mature DCs. High ICOS-L expression endows maturing pDCs with the ability to induce the differentiation of naive CD4 T cells to produce interleukin-10 (IL-10) but not the T helper (Th)2 cytokines IL-4, -5, and -13. These IL-10-producing T cells are T regulatory cells, and their generation by ICOS-L is independent of pDC-driven Th1 and Th2 differentiation, although, in the later condition, some contribution from endogenous IL-4 cannot be completely ruled out. Thus, in contrast to mDCs, pDCs are poised to express ICOS-L upon maturation, which leads to the generation of IL-10-producing T regulatory cells. Our findings demonstrate that mDC and pDCs are intrinsically different in the expression of costimulatory molecules that drive distinct types of T cell responses.
Figures
Figure 1.
ICOS-L is preferentially expressed by activated pDCs and not mDCs. Microarray gene expression profiles of B7-like molecules B7-H1, ICOS-L, B7-H3, and B7-DC in freshly isolated unstimulated (unstim) pDCs and pDCs activated by different stimuli in comparison with freshly isolated unstimulated mDCs, mDCs activated by different stimuli, and other primary immune cells of the blood. Results of the gene expression profiles are shown as the relative gene hybridization intensity level.
Figure 2.
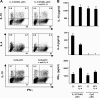
In contrast to mDCs, pDCs up-regulate ICOS-L upon activation and maintain high expression levels upon differentiation into mature DCs. (A) Surface expression of ICOS-L, CD80, and CD86 in freshly isolated untreated pDCs and pDCs activated along the IL-3–dependent pathway with IL-3 plus CD40L for 6 d or along the TLR-dependent pathway with CpG-B, CpG-A, or HSV for 1 d. Open histograms represent staining of costimulatory molecules; closed histograms represent isotype controls. (B) Mean fluorescence intensity of ICOS-L and CD86 expression in pDCs activated by CpG-B monitored at different points over a 48-h period of maturation into DCs. (C) Surface expression of ICOS-L, CD80, and CD86 on blood monocytes (Mono), monocyte-derived DCs cultured for 5 d with GM-CSF and IL-4 (immature mDC), and mature mDCs activated along the CD40L pathway (CD40L-mDC) or the TLR-dependent pathway (LPS-mDC). Results in A–C are representative of at least five independent experiments.
Figure 3.
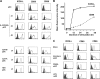
Both maturing mDCs and pDCs use CD28 costimulation to expand naive T cells. Naive peripheral CD4 T cells were cocultured with IL-3/CD40L-pDCs, CpG-pDCs, or CD40L-mDCs in the presence of neutralizing antibodies against ICOS-L (anti–ICOS-L) and against CD80 plus CD86 (anti-CD80/86) or were cocultured with isotype-matched control antibodies (−). After 6 d of culturing, T cell numbers were measured using trypan blue exclusion, and the T cell expansion was calculated. Each symbol represents an independent experiment, and horizontal bars represent the mean. P-values calculated by unpaired Student's t test are indicated.
Figure 4.
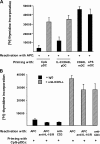
Maturing pDCs but not mDCs use ICOS-L to specifically generate T cells producing IL-10 during the induction of both Th1 and Th2 responses. Peripheral naive CD4 T cells were cocultured with CpG-pDCs, IL-3/CD40L-pDCs, LPS-mDCs, and CD40L-mDCs in the presence of neutralizing antibodies against ICOS-L (anti–ICOS-L), CD80 plus CD86 (anti-CD80/86), or isotype-matched control antibodies (−). (A) The ability of primed T cells to secrete IL-10, -4, -5, -13, IFN-γ, and TNF-α was assayed by ELISA of supernatants collected 24 h after polyclonal stimulation with anti-CD3 and -CD28 mAbs. The results are representative of six independent experiments. < indicates that the measured value was below the detection limit of the assay experiments (<20 pg/ml). T cells primed by LPS-mDCs (not depicted) secreted an equivalent cytokine profile as T cells primed by CD40L-mDCs. Error bars represent SD. (B) Intracellular production of IL-10 and IFN-γ assayed by flow cytometry 6 h after the stimulation of primed T cells with PMA and ionomycin. The percentages of each population are indicated in the plots. One of three independent experiments is shown.
Figure 5.
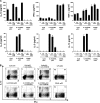
The generation of IL-10 and IL-4 are differentially regulated by ICOS-L and CD28 costimulation, respectively. Peripheral naive CD4 T cells were cultured for 6 d on parental L cells or ICOSL-L cells, which were precoated with 0.2 μg/ml anti-CD3 mAb stimulating 1 μg/ml anti-CD28 mAb or isotype-matched control antibodies were added into the culture. The ability of primed T cells to secrete IL-10, -4, and IFN-γ was assayed by ELISA of supernatants collected 24 h after polyclonal stimulation with anti-CD3 and -CD28 mAbs. The results are representative of three independent experiments. < indicates that the measured value was below the detection limit of the assay (<20 pg/ml). Error bars represent SD.
Figure 6.
Blocking of IL-4 does not inhibit IL-10 induction in Th1 cells in response to ICOS-L on maturing CpG-pDCs. Peripheral naive CD4+ T cells were cocultured with IL-3/CD40L-pDCs or CpG-pDCs in the presence of neutralizing antibodies against IL-4 or isotype-matched control antibodies (−). (A) Intracellular production of IL-10 and IFN-γ (top and bottom plots) as well as IL-4 and IFN-γ (middle plots) was assayed by flow cytometry 6 h after the stimulation of primed T cells with PMA and ionomycin. The percentages of each population are indicated in the plot. (B) The ability of primed T cells to secrete IL-10, -4, or IFN-γ was assayed by ELISA of supernatants collected 24 h after polyclonal stimulation with anti-CD3 and -CD28 mAbs. One representative experiment of four independent experiments is shown. < indicates that the measured value was below the detection limit of the assay (<20 pg/ml). Error bars represent SD.
Figure 7.
IL-10–producing T cells generated by ICOS-L on maturing pDCs are anergic. (A) CD4 T cells primed by CpG-pDCs, IL-3/CD40L- pDCs, LPS-mDCs, and CD40L-mDCs in the presence of neutralizing anti–ICOS-L mAbs (hatched bars) or isotype-matched control antibodies (black bars) were collected after 6 d and reactivated with allogeneic monocytes from the same donor whose cells were used for priming. The ability of primed T cells to mount a proliferative response was assessed by 3[H]thymidine incorporation after 2 d. (B) CD4 T cells primed by CpG-pDCs in the presence of neutralizing anti–ICOS-L mAbs (hatched bars) or isotype-matched control antibodies (black bars) were either reactivated by allogeneic monocytes (APC) or low doses of anti-CD3 (1 μg/ml; plate bound) plus or minus neutralizing mAbs to IL-10 and -10R. (A and B) Results are expressed as mean counts per minute + SD (error bars) of triplicate wells and are representative of three independent experiments.
Figure 8.
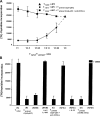
IL-10–producing T cells generated by ICOS-L on maturing pDCs are T regulatory cells. (A) The capacity of primed T cells to suppress primary T cell responses was tested by stimulating naive CD4+ T cells with allogeneic monocytes (APC) in the presence of decreasing numbers of syngeneic T cells primed by CpG-pDCs alone (circles) or CpG-pDCs in the presence of anti–ICOS-L mAbs (squares). 3[H]thymidine incorporation was assessed after 6 d. Results are representative of three independent experiments. Importantly, the maximal proliferation of restimulated T cells primed with CpG-DCs or CpG-DCs plus anti–ICOS-L alone after 6 d was <5,000 cpm. (B) Naive CD4+ T cells were cultured with allogeneic monocytes alone (A), monocytes plus T cells primed by CpG-pDCs (B), monocytes plus T cells primed by CpG-pDCs in the presence of anti–ICOS-L mAbs (C), and monocytes plus T cells primed by maturing mDCs (D). The role of IL-10 in the suppressive activity of primed T cells was determined by adding anti–IL-10/IL-10R mAbs (anti-IL10/R) or recombinant human IL-10 to the suppression assay. The results are representative of five experiments. Similar to T cells primed by CpG-pDCs, T cells primed by IL-3/CD40L-pDCs suppressed primary T cell responses (not depicted). (A and B) Results are expressed as mean counts per minute + SD (error bars) of triplicate wells.
Similar articles
- Plasmacytoid dendritic cells have a cytokine-producing capacity to enhance ICOS ligand-mediated IL-10 production during T-cell priming.
Ogata M, Ito T, Shimamoto K, Nakanishi T, Satsutani N, Miyamoto R, Nomura S. Ogata M, et al. Int Immunol. 2013 Mar;25(3):171-82. doi: 10.1093/intimm/dxs103. Epub 2012 Nov 2. Int Immunol. 2013. PMID: 23125331 - Streptococcus pyogenes activates human plasmacytoid and myeloid dendritic cells.
Veckman V, Julkunen I. Veckman V, et al. J Leukoc Biol. 2008 Feb;83(2):296-304. doi: 10.1189/jlb.0707457. Epub 2007 Oct 26. J Leukoc Biol. 2008. PMID: 17965337 - Eminent role of ICOS costimulation for T cells interacting with plasmacytoid dendritic cells.
Janke M, Witsch EJ, Mages HW, Hutloff A, Kroczek RA. Janke M, et al. Immunology. 2006 Jul;118(3):353-60. doi: 10.1111/j.1365-2567.2006.02379.x. Immunology. 2006. PMID: 16827896 Free PMC article. - Dendritic cells from bench to bedside and back.
Adema GJ. Adema GJ. Immunol Lett. 2009 Feb 21;122(2):128-30. doi: 10.1016/j.imlet.2008.11.017. Epub 2008 Dec 31. Immunol Lett. 2009. PMID: 19121337 Review. - Development of dendritic cell system.
Wu L, Dakic A. Wu L, et al. Cell Mol Immunol. 2004 Apr;1(2):112-8. Cell Mol Immunol. 2004. PMID: 16212897 Review.
Cited by
- Human dendritic cell subsets: An updated view of their ontogeny and functional specialization.
Segura E. Segura E. Eur J Immunol. 2022 Nov;52(11):1759-1767. doi: 10.1002/eji.202149632. Epub 2022 Mar 11. Eur J Immunol. 2022. PMID: 35187651 Free PMC article. Review. - Virus-like particles (VLP) in prophylaxis and immunotherapy of allergic diseases.
Klimek L, Kündig T, Kramer MF, Guethoff S, Jensen-Jarolim E, Schmidt-Weber CB, Palomares O, Mohsen MO, Jakob T, Bachmann M. Klimek L, et al. Allergo J Int. 2018;27(8):245-255. doi: 10.1007/s40629-018-0074-y. Epub 2018 Jul 9. Allergo J Int. 2018. PMID: 30546996 Free PMC article. Review. - Why functional pre-erythrocytic and bloodstage malaria vaccines fail: a meta-analysis of fully protective immunizations and novel immunological model.
Guilbride DL, Gawlinski P, Guilbride PD. Guilbride DL, et al. PLoS One. 2010 May 19;5(5):e10685. doi: 10.1371/journal.pone.0010685. PLoS One. 2010. PMID: 20502667 Free PMC article. - IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection.
Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, de Souza JB, Riley EM. Couper KN, et al. PLoS Pathog. 2008 Feb 29;4(2):e1000004. doi: 10.1371/journal.ppat.1000004. PLoS Pathog. 2008. PMID: 18401464 Free PMC article. - Dendritic cells in liver transplantation immune response.
Du X, Li M, Huan C, Lv G. Du X, et al. Front Cell Dev Biol. 2023 Oct 13;11:1277743. doi: 10.3389/fcell.2023.1277743. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37900282 Free PMC article. Review.
References
- Liu, Y.J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 106:259–262. - PubMed
- Colonna, M., G. Trinchieri, and Y.J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219–1226. - PubMed
- Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393. - PubMed
- Ito, T., H. Kanzler, O. Duramad, W. Cao, and Y.J. Liu. 2006. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 107:2423–2431. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous