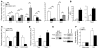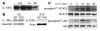Obesity induces a phenotypic switch in adipose tissue macrophage polarization - PubMed (original) (raw)
Obesity induces a phenotypic switch in adipose tissue macrophage polarization
Carey N Lumeng et al. J Clin Invest. 2007 Jan.
Abstract
Adipose tissue macrophages (ATMs) infiltrate adipose tissue during obesity and contribute to insulin resistance. We hypothesized that macrophages migrating to adipose tissue upon high-fat feeding may differ from those that reside there under normal diet conditions. To this end, we found a novel F4/80(+)CD11c(+) population of ATMs in adipose tissue of obese mice that was not seen in lean mice. ATMs from lean mice expressed many genes characteristic of M2 or "alternatively activated" macrophages, including Ym1, arginase 1, and Il10. Diet-induced obesity decreased expression of these genes in ATMs while increasing expression of genes such as those encoding TNF-alpha and iNOS that are characteristic of M1 or "classically activated" macrophages. Interestingly, ATMs from obese C-C motif chemokine receptor 2-KO (Ccr2-KO) mice express M2 markers at levels similar to those from lean mice. The antiinflammatory cytokine IL-10, which was overexpressed in ATMs from lean mice, protected adipocytes from TNF-alpha-induced insulin resistance. Thus, diet-induced obesity leads to a shift in the activation state of ATMs from an M2-polarized state in lean animals that may protect adipocytes from inflammation to an M1 proinflammatory state that contributes to insulin resistance.
Figures
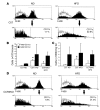
Figure 1. Accumulation of F4/80+CD11c+ ATMs in adipose tissue in obese mice.
(A) Analysis of SVF cells for F4/80 and CD11c. Epididymal fat pads from age-matched male C57BL/6 (C57) mice on ND or HFD (n = 3 mice, each condition) were dissected and separated into adipocyte and SVF populations. SVF cells were stained with antibodies against F4/80, CD11c, and isotype controls (open) and analyzed by flow cytometry. Samples were gated for F4/80+ cells and examined for coexpression of CD11c (lower panels). Data from a representative experiment are shown. The percentage of CD11c+ cells within the F4/80+ ATM population is indicated for each condition. (B and C) Quantitation of CD11c+ and CD11c– ATM subpopulations in epididymal fat pads. Flow cytometry was used to assess the percentages of F4/80+CD11c+ and F4/80+CD11c– ATMs in SVF samples from ND- and HFD-fed C57BL/6 mice and HFD-fed CCR2KO mice (n = 3–4 mice per condition). Data are presented as total number of cells per mouse for each ATM subtype (B) and as cell counts normalized to cell number and fat pad weight (C). Data are presented as mean ± SD *P < 0.05 versus ND. (D) Analysis of CD11c expression in ATMs isolated from epididymal fat pads from male CCR2KO mice on a C57BL/6 background on ND or HFD. Cell isolation and flow cytometry were performed as described for A.
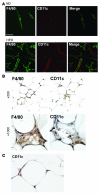
Figure 2. CD11c+ ATMs in SVF cultures and in adipose tissue from obese mice.
(A) Identification of F4/80+ and CD11c+ ATMs by immunofluorescence microscopy. Epididymal fat pads from ND and HFD mice were separated into adipocyte and SVF fractions. SVF cells were plated onto glass coverslips and cultured overnight prior to fixation. Cells were stained with antibodies against F4/80 (left) and CD11c (middle) and imaged by confocal microscopy to identify surface markers to confirm the presence of CD11c+ cells only in the SVF from HFD-fed animals. Similar results were obtained for 3 independent sets of cultures. (B) Immunohistochemical localization of CD11c+ in adipose tissue. Consecutive sections from epididymal fat pads from obese C57BL/6 mice were stained with anti-F4/80 (left panels) and anti-CD11c antibodies (right panels), followed by colorimetric detection (brown). Sections were counterstained with hematoxylin (blue) and images taken at low (×200) and high magnification (×1,000). (C) In obese mice, CD11c+ cells were also detected surrounding normal-appearing adipocytes in the absence of crownlike macrophage clusters (×1,000 magnification).
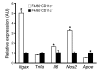
Figure 3. Increased inflammatory gene expression in F4/80+CD11c+ ATMs.
SVF cells were isolated from HFD-fed male C57BL/6 mice (n = 3) and stained for F4/80 and CD11c. F4/80+CD11c+ and F4/80+CD11c– cells were isolated by FACS and total RNA isolated. Expression of Itgax (CD11c), Tnfa (TNF-α), Il6 (IL-6), Nos2 (iNOS), and Apoe (apoE) was analyzed by real-time RT-PCR in F4/80+CD11c+ (white bars) and F4/80+CD11c– (black bars) ATMs. Data are expressed as mean ± SD. *P < 0.05.
Figure 4. Increased expression of markers of alternatively activated (M2) macrophages in ATMs from lean ND-fed mice.
(A and B) Gene expression in ATMs from ND and HFD mice. F4/80+CD11b+ ATMs were isolated from ND C57BL/6 (white bars), HFD C57BL/6 (black bars), and HFD CCR2KO mice (gray bars) (n = 3 pools of mice for each) and analyzed by real-time RT-PCR for expression of M2 macrophage–specific genes (A) and proinflammatory genes (B). Data are expressed as mean ± SD. *P < 0.05. (C and D) SVF was isolated from ND (white bars) and HFD (black bars) mice (n = 2–3 mice per condition) and analyzed by real-time RT-PCR for expression of M2 macrophage markers (C) and proinflammatory genes (D). (E) Ym1 protein expression in the SVF. Lysates from SVF isolated from ND and HFD mice were immunoblotted for Ym1 (left). CD11b+ ATMs were separated from CD11b– cells in the SVF and lysates prepared for immunoblotting, which demonstrated Ym1 expression in the macrophage fraction (right). Macrophage marker CD68 controlled for the purification protocol. Similar results were obtained in a duplicate experiment. (F) Arginase activity in adipose tissue from ND and HFD mice. Epididymal fat pads from ND- (white bars) and HFD-fed (black bars) mice were separated into adipocyte and SVF fractions and lysates prepared. Arginase activity was assessed by an assay of urea production from arginine substrate and was normalized to protein concentration. Reactions were performed in triplicate. Data are expressed as mean ± SD. *P < 0.05. Similar results were obtained for 3 separate sets of mice.
Figure 5. IL-10 signaling in adipocytes.
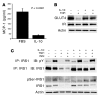
(A) Expression of IL-10 receptor in adipose tissue. Immunoblots of lysates from adipose tissue (Ad), lung (Lu), and spleen (Sp) from mice probed with IL-10 receptor α (IL-10Rα) antibodies. (B) Expression of IL-10 receptor in adipocytes and not the SVF from adipose tissue. Epididymal fat pads from ND- and HFD-fed mice were collected and separated into SVF and adipocyte fractions. Lysates were prepared and immunoblotted with anti–IL-10 receptor antibodies and loading controls. (C) IL-10 treatment of adipocytes activates STAT3 and Akt. Differentiated 3T3-L1 adipocytes were stimulated with IL-10 (20 ng/ml) for the indicated times and lysates prepared. Immunoblots were probed with antibodies against phosphoY705-STAT3 and phosphoS473-Akt and STAT3 and Akt as loading controls. Experiments were repeated twice, and results of a representative experiment are shown.
Figure 6. IL-10 prevents the effects of TNF-α on adipocytes.
(A) IL-10 decreases MCP-1 secretion by adipocytes. 3T3-L1 adipocytes were treated with media with or without IL-10 (20 ng/ml) for 16 hours. Conditioned media was then removed and assayed for MCP-1 levels by ELISA. n = 5 independent samples per condition. Data are expressed as mean ± SD. (B) IL-10 protects adipocytes from TNF-α–induced downregulation of insulin receptor and glucose transporter 4 (GLUT4) expression. 3T3-L1 cells were treated with or without IL-10 for 24 hours prior to treatment with or without TNF-α (17 ng/ml for 6 hours). Lysates were prepared and immunoblots probed with antibodies against the insulin receptor (IR) and GLUT4. (C) IL-10 maintains IRS levels despite treatment with TNF-α. Adipocytes were treated as described for B and lysates examined for IRS1 tyrosine phosphorylation induced by insulin (INS; 100 nM for 5 minutes) after immunoprecipitation of IRS1. IRS serine phosphorylation at Ser307 was evaluated using specific antibodies.
Figure 7. IL-10 prevents the effects of TNF-α on blocking insulin-stimulated glucose uptake in adipocytes.
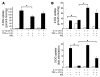
(A) Pretreatment of adipocytes with IL-10 blocks TNF-α effects on glucose uptake. 3T3-L1 adipocytes were treated with IL-10 (20 ng/ml) for 24 hours prior to treatment with TNF-α (17 ng/ml) for 3 hours. 2-Deoxyglucose (2-DG) uptake was assessed after 30 minutes without (white bars) or with (black bars) insulin (100 nM) stimulation. Data are expressed as mean ± SD of triplicate experiments repeated 3 times. *P < 0.05. (B) Insulin-stimulated glucose uptake in adipocytes chronically treated with IL-10 and TNF-α. Differentiated 3T3-L1 adipocytes were treated with low-dose TNF-α (3 ng/ml) for 72 hours in the presence or absence of IL-10 (20 ng/ml). After insulin stimulation (black bars), 2-DG uptake (upper panel) and fold change in glucose uptake (lower panel) were assessed, and the results demonstrated that IL-10 blocks the effects of TNF-α. Data are expressed as mean ± SD of triplicate experiments repeated 2 times. *P < 0.05.
Figure 8. Proposed model for ATM polarization and its function in adipose tissue with progressive obesity.
In lean, insulin-sensitive states, ATMs are polarized toward an M2 state with IL-10 and arginase expression. Early during HFD treatment, adipocytes undergo hypertrophy, releasing chemokines that induce recruitment of M1-polarized ATMs with low IL-10 expression and increased iNOS and TNF-α production. In these early stages of mild obesity with retained insulin sensitivity, M2-polarized resident ATMs are able to partially protect adipocytes from these inflammatory factors and may block M1 polarization. With increased adiposity, recruited CCR2+ ATMs form crownlike structures (CLS) and overwhelm the protective effects of M2 macrophages, leading to a dominant role for TNF-α and iNOS. These factors generate insulin resistance in adipocytes, activate JNK and NF-κB, alter adipokine secretion, and lead to excess circulating levels of free fatty acids due to adipocyte lipolysis and impaired lipogenesis.
Comment in
- Macrophage heterogeneity and tissue lipids.
Gordon S. Gordon S. J Clin Invest. 2007 Jan;117(1):89-93. doi: 10.1172/JCI30992. J Clin Invest. 2007. PMID: 17200712 Free PMC article.
Similar articles
- Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity.
Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Lumeng CN, et al. Diabetes. 2007 Jan;56(1):16-23. doi: 10.2337/db06-1076. Diabetes. 2007. PMID: 17192460 - Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice.
Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Fujisaka S, et al. Diabetes. 2009 Nov;58(11):2574-82. doi: 10.2337/db08-1475. Epub 2009 Aug 18. Diabetes. 2009. PMID: 19690061 Free PMC article. - Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes.
Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Lumeng CN, et al. Diabetes. 2008 Dec;57(12):3239-46. doi: 10.2337/db08-0872. Epub 2008 Oct 1. Diabetes. 2008. PMID: 18829989 Free PMC article. - Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states.
Morris DL, Singer K, Lumeng CN. Morris DL, et al. Curr Opin Clin Nutr Metab Care. 2011 Jul;14(4):341-6. doi: 10.1097/MCO.0b013e328347970b. Curr Opin Clin Nutr Metab Care. 2011. PMID: 21587064 Free PMC article. Review. - The role of adipose tissue M1/M2 macrophages in type 2 diabetes mellitus.
Fujisaka S. Fujisaka S. Diabetol Int. 2020 Dec 15;12(1):74-79. doi: 10.1007/s13340-020-00482-2. eCollection 2021 Jan. Diabetol Int. 2020. PMID: 33479582 Free PMC article. Review.
Cited by
- Novel perspectives on the link between obesity and cancer risk: from mechanisms to clinical implications.
Shi X, Jiang A, Qiu Z, Lin A, Liu Z, Zhu L, Mou W, Cheng Q, Zhang J, Miao K, Luo P. Shi X, et al. Front Med. 2024 Nov 14. doi: 10.1007/s11684-024-1094-2. Online ahead of print. Front Med. 2024. PMID: 39542988 Review. - Association between sagittal abdominal diameter-to-height ratio and all-cause mortality among adults in the United States: a longitudinal study.
Gu X, Gao P, Zhu F, Shen Y, Lu L. Gu X, et al. Arch Public Health. 2024 Nov 14;82(1):213. doi: 10.1186/s13690-024-01443-w. Arch Public Health. 2024. PMID: 39538327 Free PMC article. - Body Composition and Senescence: Impact of Polyphenols on Aging-Associated Events.
Santos TWD, Pereira QC, Fortunato IM, Oliveira FS, Alvarez MC, Ribeiro ML. Santos TWD, et al. Nutrients. 2024 Oct 25;16(21):3621. doi: 10.3390/nu16213621. Nutrients. 2024. PMID: 39519454 Free PMC article. Review. - Time-Restricted Feeding Attenuates Adipose Tissue Inflammation and Fibrosis in Mice Under Chronic Light Exposure.
Nah J, Yun N, Yoo H, Park S, Pae M. Nah J, et al. Int J Mol Sci. 2024 Oct 26;25(21):11524. doi: 10.3390/ijms252111524. Int J Mol Sci. 2024. PMID: 39519077 Free PMC article. - Impact of Conjugated Linoleic Acid on Obesity and Its Association with Macrophage Recruitment: Experimental and Immunohistochemical Study.
Abdou AG, Bendary MA, Abdou SE, Amer GS. Abdou AG, et al. J Microsc Ultrastruct. 2022 Nov 14;12(3):142-147. doi: 10.4103/jmau.jmau_25_22. eCollection 2024 Jul-Sep. J Microsc Ultrastruct. 2022. PMID: 39507643 Free PMC article.
References
- Greenberg A.S., Obin M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006;83:461S–465S. - PubMed
- Cancello R., et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK60591/DK/NIDDK NIH HHS/United States
- T32 HD 007513-07/HD/NICHD NIH HHS/United States
- K12 HD028820-15/HD/NICHD NIH HHS/United States
- T32 HD007513/HD/NICHD NIH HHS/United States
- K12 HD028820/HD/NICHD NIH HHS/United States
- R01 DK060591/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous