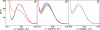Direct observation in solution of a preexisting structural equilibrium for a mutant of the allosteric aspartate transcarbamoylase - PubMed (original) (raw)
Direct observation in solution of a preexisting structural equilibrium for a mutant of the allosteric aspartate transcarbamoylase
Luc Fetler et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Many signaling and metabolic pathways rely on the ability of some of the proteins involved to undergo a substrate-induced transition between at least two structural states. Among the various models put forward to account for binding and activity curves of those allosteric proteins, the Monod, Wyman, and Changeux model for allostery theory has certainly been the most influential, although a central postulate, the preexisting equilibrium between the low-activity, low-affinity quaternary structure and the high-activity, high-affinity quaternary structure states in the absence of substrates, has long awaited direct experimental substantiation. Upon substrate binding, allosteric Escherichia coli aspartate transcarbamoylase adopts alternate quaternary structures, stabilized by a set of interdomain and intersubunit interactions, which are readily differentiated by their solution x-ray scattering curves. Disruption of a salt link, which is observed only in the low-activity, low-affinity quaternary structure, between Lys-143 of the regulatory chain and Asp-236 of the catalytic chain yields a mutant enzyme that is in a reversible equilibrium between at least two states in the absence of ligand, a major tenet of the Monod, Wyman, and Changeux model. By using this mutant as a magnifying glass of the structural effect of ligand binding, a comparative analysis of the binding of carbamoyl phosphate (CP) and analogs points out the crucial role of the amine group of CP in facilitating the transition toward the high-activity, high-affinity quaternary state. Thus, the cooperative binding of aspartate in aspartate transcarbamoylase appears to result from the combination of the preexisting quaternary structure equilibrium with local changes induced by CP binding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
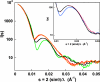
Fig. 1.
Solution x-ray scattering spectra of wild-type and D236A ATCase. Green, unliganded wild-type enzyme; black, unliganded D236A ATCase; red, wild-type enzyme in the presence of a 2-fold molar excess of PALA; orange, D236A ATCase in the presence of a 2-fold molar excess of PALA. (Inset) Solution x-ray scattering spectrum of the D236A ATCase with experimental error bars and linear combinations of wild-type enzyme extreme patterns. Black, unliganded D236A enzyme; magenta, 56% T-44% R; blue, 76% T-24% R.
Fig. 2.
Solution x-ray scattering spectra of D236A ATCase in the presence of nucleotide effectors. (A) pH 8.3. Black, unliganded D236A ATCase; brown, D236A ATCase + 5 mM ATP; blue, D236A ATCase + 5 mM CTP; green, unliganded wild-type ATCase (T-state). (B) pH 7. Black, unliganded D236A ATCase; green, D236A ATCase + 5 mM Mg-ATP; red, PALA-bound D236A ATCase + 5 mM Mg-ATP; blue, PALA-bound wild-type ATCase + 5 mM Mg-ATP.
Fig. 3.
Effect of temperature on both wild-type and D236A unliganded ATCases. (A) SAXS patterns of unliganded wild-type ATCase recorded at temperatures ranging from 4°C (dark blue) to 60°C (red). (B) SAXS patterns of unliganded D236A ATCase at temperatures ranging from 6°C (dark blue) to 45°C (red). (C) Reversibility of the temperature effect on the unliganded D236A ATCase. Scattering patterns recorded at 15°C (blue) and 20°C (orange) during the heating (continuous line) and cooling (dashed line) phases.
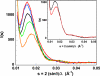
Fig. 4.
Solution x-ray scattering spectra of the D236A ATCase in the presence of the first substrate, CP, and its analogs. Black, unliganded D236A ATCase; blue, D236A ATCase + 5 mM CP; red, D236A ATCase + 2-fold molar excess of PALA; green, D236A ATCase + 5 mM _N_-methyl-carbamoyl phosphate; orange, D236A ATCase + 5 mM acetyl phosphate. (Inset) black, unliganded D236A ATCase; red, D236A ATCase + 100 mM aspartate.
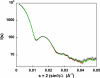
Fig. 5.
Fitting of the unliganded D236A ATCase scattering pattern. Black, experimental SAXS pattern of the unliganded D236A ATCase with experimental error bars; red, linear combination of 62% T-38% Mg-ATP-bound D236A ATCase; green, linear combination of 63% T-37% Rsol+MgATP.
Similar articles
- Glu-50 in the catalytic chain of Escherichia coli aspartate transcarbamoylase plays a crucial role in the stability of the R quaternary structure.
Tauc P, Keiser RT, Kantrowitz ER, Vachette P. Tauc P, et al. Protein Sci. 1994 Nov;3(11):1998-2004. doi: 10.1002/pro.5560031112. Protein Sci. 1994. PMID: 7703847 Free PMC article. - A single amino acid substitution in the active site of Escherichia coli aspartate transcarbamoylase prevents the allosteric transition.
Stieglitz KA, Pastra-Landis SC, Xia J, Tsuruta H, Kantrowitz ER. Stieglitz KA, et al. J Mol Biol. 2005 Jun 3;349(2):413-23. doi: 10.1016/j.jmb.2005.03.073. Epub 2005 Apr 9. J Mol Biol. 2005. PMID: 15890205 Free PMC article. - Weakening of the interface between adjacent catalytic chains promotes domain closure in Escherichia coli aspartate transcarbamoylase.
Baker DP, Fetler L, Keiser RT, Vachette P, Kantrowitz ER. Baker DP, et al. Protein Sci. 1995 Feb;4(2):258-67. doi: 10.1002/pro.5560040212. Protein Sci. 1995. PMID: 7757014 Free PMC article. - From feedback inhibition to allostery: the enduring example of aspartate transcarbamoylase.
Gerhart J. Gerhart J. FEBS J. 2014 Jan;281(2):612-20. doi: 10.1111/febs.12483. Epub 2013 Sep 5. FEBS J. 2014. PMID: 23953008 Review. - Allostery and cooperativity in Escherichia coli aspartate transcarbamoylase.
Kantrowitz ER. Kantrowitz ER. Arch Biochem Biophys. 2012 Mar 15;519(2):81-90. doi: 10.1016/j.abb.2011.10.024. Epub 2011 Dec 16. Arch Biochem Biophys. 2012. PMID: 22198283 Free PMC article. Review.
Cited by
- Crystallographic and kinetic evidence of allostery in a trypsin-like protease.
Niu W, Chen Z, Gandhi PS, Vogt AD, Pozzi N, Pelc LA, Zapata F, Di Cera E. Niu W, et al. Biochemistry. 2011 Jul 26;50(29):6301-7. doi: 10.1021/bi200878c. Epub 2011 Jun 30. Biochemistry. 2011. PMID: 21707111 Free PMC article. - Solution structural ensembles of substrate-free cytochrome P450(cam).
Asciutto EK, Young MJ, Madura J, Pochapsky SS, Pochapsky TC. Asciutto EK, et al. Biochemistry. 2012 Apr 24;51(16):3383-93. doi: 10.1021/bi300007r. Epub 2012 Apr 10. Biochemistry. 2012. PMID: 22468842 Free PMC article. - Structural Basis of Sequential and Concerted Cooperativity.
Morea V, Angelucci F, Tame JRH, Di Cera E, Bellelli A. Morea V, et al. Biomolecules. 2022 Nov 7;12(11):1651. doi: 10.3390/biom12111651. Biomolecules. 2022. PMID: 36359000 Free PMC article. - Influence of the cosolute environment on IgG solution structure analyzed by small-angle X-ray scattering.
Lilyestrom WG, Shire SJ, Scherer TM. Lilyestrom WG, et al. J Phys Chem B. 2012 Aug 16;116(32):9611-8. doi: 10.1021/jp303839t. Epub 2012 Aug 3. J Phys Chem B. 2012. PMID: 22827493 Free PMC article. - BEES: Bayesian Ensemble Estimation from SAS.
Bowerman S, Curtis JE, Clayton J, Brookes EH, Wereszczynski J. Bowerman S, et al. Biophys J. 2019 Aug 6;117(3):399-407. doi: 10.1016/j.bpj.2019.06.024. Epub 2019 Jul 18. Biophys J. 2019. PMID: 31337549 Free PMC article.
References
- Changeux JP, Edelstein SJ. Science. 2005;308:1424–1428. - PubMed
- Monod J, Wyman J, Changeux JP. J Mol Biol. 1965;12:88–118. - PubMed
- Perutz MF. Q Rev Biophys. 1989;22:139–237. - PubMed
- Hervé G. Allosteric Enzymes. Boca Raton, FL: CRC; 1989.
- Iwata S, Kamata K, Yoshida S, Minowa T, Ohta T. Nat Struct Biol. 1994;1:176–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous