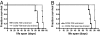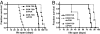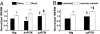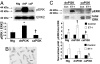Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy - PubMed (original) (raw)
. 2007 Jan 9;104(2):612-7.
doi: 10.1073/pnas.0606663104. Epub 2007 Jan 3.
Fatemeh Amirahmadi, Elizabeth A Woodcock, Martina Schinke-Braun, Russell D Bouwman, Kimberly A Hewitt, Janelle P Mollica, Li Zhang, Yunyu Zhang, Tetsuo Shioi, Antje Buerger, Seigo Izumo, Patrick Y Jay, Garry L Jennings
Affiliations
- PMID: 17202264
- PMCID: PMC1766433
- DOI: 10.1073/pnas.0606663104
Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy
Julie R McMullen et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Physical activity protects against cardiovascular disease, and physiological cardiac hypertrophy associated with regular exercise is usually beneficial, in marked contrast to pathological hypertrophy associated with disease. The p110alpha isoform of phosphoinositide 3-kinase (PI3K) plays a critical role in the induction of exercise-induced hypertrophy. Whether it or other genes activated in the athlete's heart might have an impact on cardiac function and survival in a setting of heart failure is unknown. To examine whether progressive exercise training and PI3K(p110alpha) activity affect survival and/or cardiac function in two models of heart disease, we subjected a transgenic mouse model of dilated cardiomyopathy (DCM) to swim training, genetically crossed cardiac-specific transgenic mice with increased or decreased PI3K(p110alpha) activity to the DCM model, and subjected PI3K(p110alpha) transgenics to acute pressure overload (ascending aortic constriction). Life-span, cardiac function, and molecular markers of pathological hypertrophy were examined. Exercise training and increased cardiac PI3K(p110alpha) activity prolonged survival in the DCM model by 15-20%. In contrast, reduced PI3K(p110alpha) activity drastically shortened lifespan by approximately 50%. Increased PI3K(p110alpha) activity had a favorable effect on cardiac function and fibrosis in the pressure-overload model and attenuated pathological growth. PI3K(p110alpha) signaling negatively regulated G protein-coupled receptor stimulated extracellular responsive kinase and Akt (via PI3K, p110gamma) activation in isolated cardiomyocytes. These findings suggest that exercise and enhanced PI3K(p110alpha) activity delay or prevent progression of heart disease, and that supraphysiologic activity can be beneficial. Identification of genes important for hypertrophy in the athlete's heart could offer new strategies for treating heart failure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Swim training prolongs the lifespan of mice with DCM. Kaplan–Meier survival curves of untrained and exercise-trained DCM-TG9 mice. (A) Males, n = 4 in each group; curve comparison, P < 0.007. (B) Females, untrained n = 5, exercise-trained n = 3, P < 0.02).
Fig. 2.
Swim training reduced the expression of fetal genes in mice with DCM. (A) Representative Northern blot showing total RNA from ventricles of untrained (un) and exercise trained (ex) Ntg and DCM-TG9 mice. (B) Quantitative analysis of fetal gene expression (fold change). GAPDH was used to normalize for loading of RNA. Mean values for Ntg untrained (un) mice were normalized to 1 (n = 3 or 4 in each group). ∗, P < 0.05 vs. Ntg untrained mice. †, P < 0.05 vs. DCM-TG9 untrained mice. α-sk actin, α-skeletal actin.
Fig. 3.
Enhanced PI3K activity improves the lifespan of mice with DCM. Survival curves of male (A) and female (B) DCM-TG9 mice (n = 12 and 5, respectively) with and without increased PI3K activity (DCM-TG9-caPI3K mice; n = 6 and 3, respectively) or decreased PI3K activity (DCM-TG9-dnPI3K mice; n = 8 and 3, respectively). Curve comparison shows for DCM-TG9 vs. DCM-TG9-caPI3K mice, male, P < 0.0002; female, P < 0.02 and for DCM-TG9 vs. DCM-TG9-dnPI3K mice, male, P < 0.0001; female, P < 0.005.
Fig. 4.
Hypertrophic responses of PI3K-Tg mice to pressure overload and swim training. (A) Response of Ntg, dnPI3K, and caPI3K mice to aortic banding (band) for 1 week or the sham operation. HW/BW ratios are normalized to Ntg sham. Numbers for each group are shown in Table 2. *, P < 0.0001 vs. sham of the same genotype. †, P < 0.05 vs. Ntg sham. ‡, P < 0.05 vs. sham of the same genotype. (B) Response of Ntg and caPI3K mice to swim training for 4 weeks. HW/BW ratios are normalized to Ntg untrained mice. Numbers for each group are shown in
SI Table 3
. *, P < 0.05 vs. untrained mice of the same genotype. †, P < 0.05 vs. Ntg untrained mice. ‡, P < 0.05 vs. Ntg exercise-trained mice.
Fig. 5.
Fetal gene expression in PI3K-Tg mice subjected to pressure overload. (Left) Expression levels of BNP, ANP, α-skeletal actin (α-sk actin), and SERCA2a were examined in total RNA from ventricles of sham (Sh) and aortic-banded (B) mice by Northern blotting (representative blot). GAPDH was used to normalize for RNA loading. (Right) Quantitative analysis (fold change). Values were normalized to Ntg sham, n = 2–3 in the sham groups, n = 3 in each banded group. *, P < 0.05 vs. sham of the same genotype. †, P < 0.08 vs. Ntg sham. ‡, P < 0.05 vs. dnPI3K sham. §, P < 0.05 vs. Ntg sham. ¶, P < 0.05 vs. all other banded groups. ‖, P < 0.05 vs. Ntg band. **, P < 0.05 vs. dnPI3K band.
Fig. 6.
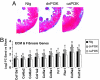
Effect of PI3K activity on cardiac fibrosis. (A) Histological sections from the LV wall of Ntg and PI3K-Tg mice subjected to aortic banding. Fibrosis is blue on Masson's trichrome stain. (B) Gene expression changes measured by microarray [log 2 fold change (FC) of band vs. sham] of extracellular matrix (ECM)- and fibrosis-related genes. Col, procollagen types: 15a1, XV; 6a2 and 6a3, VI alpha 2 and 3; 1a1 and 1a2, I alpha 1 and 2; 5a1 and 5a2, V alpha 1 and 2; 8a1, VIII alpha 1; Fn1, fibronectin 1; Fbn1, fibrillin 1. n = 3 in each group. *, adjusted P < 0.05 for band vs. sham. †, adjusted P < 0.001 for band vs. sham. ‡, adjusted P < 0.0001 for band vs. sham.
Fig. 7.
ERK1/2 and Akt activation in heart lysates and/or isolated cardiomyocytes from PI3K-Tg mice. (A) (Upper) Western blot showing pERK1/2 in heart lysates (100 μg protein) from Ntg, dnPI3K (dnP), and caPI3K (caP) mice. pERK1/2 was normalized to ERK2. (Lower) Quantitative analysis. Mean values for Ntg mice were normalized to 1, n = 3 in each group. *, P < 0.05 vs. Ntg mice. †, P < 0.05 vs. dnPI3K mice. (B) Photograph of quiescent rod-shaped cardiomyocytes. (Scale bar: 0.1 mm.) (C) ERK1/2 and Akt activation in myocytes from Ntg, dnPI3K, and caPI3K mice to ET-1 stimulation vs. no stimulation (control, Con). (Top) Western blot shows pERK1/2 in myocytes (15 μg protein) from dnPI3K and caPI3K mice. (Middle) Quantitative analysis of pERK1/2 normalized to total ERK1/2. (Bottom) Quantitative analysis of pAkt normalized to Akt or GAPDH. Control values from each genotype were normalized to 1, n = 3 in each group. *, P < 0.08 vs. Ntg control myocytes. †, P < 0.05 vs. dnPI3K control myocytes. ‡, P < 0.05 vs. dnPI3K myocytes stimulated with ET-1. §, P < 0.05 vs. Ntg myocytes stimulated with ET-1 (unpaired t test).
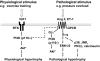
Fig. 8.
Schematic illustrates physiological and pathological signaling cascades. A physiological stimulus acting via the IGF1-PI3K(p110α) pathway may inhibit signaling molecules downstream of GPCRs activated by pathological stimuli. Ang II, angiotensin II. Dotted line indicates it may not be a direct interaction. * indicates differential regulation of Akt by GPCRs vs. RTKs (31).
Similar articles
- Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy.
McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. McMullen JR, et al. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12355-60. doi: 10.1073/pnas.1934654100. Epub 2003 Sep 24. Proc Natl Acad Sci U S A. 2003. PMID: 14507992 Free PMC article. - Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction.
Weeks KL, Gao X, Du XJ, Boey EJ, Matsumoto A, Bernardo BC, Kiriazis H, Cemerlang N, Tan JW, Tham YK, Franke TF, Qian H, Bogoyevitch MA, Woodcock EA, Febbraio MA, Gregorevic P, McMullen JR. Weeks KL, et al. Circ Heart Fail. 2012 Jul 1;5(4):523-34. doi: 10.1161/CIRCHEARTFAILURE.112.966622. Epub 2012 Jun 15. Circ Heart Fail. 2012. PMID: 22705768 - Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110α) signaling.
De Blasio MJ, Huynh K, Qin C, Rosli S, Kiriazis H, Ayer A, Cemerlang N, Stocker R, Du XJ, McMullen JR, Ritchie RH. De Blasio MJ, et al. Free Radic Biol Med. 2015 Oct;87:137-47. doi: 10.1016/j.freeradbiomed.2015.04.028. Epub 2015 Apr 30. Free Radic Biol Med. 2015. PMID: 25937176 - The IGF1-PI3K-Akt Signaling Pathway in Mediating Exercise-Induced Cardiac Hypertrophy and Protection.
Weeks KL, Bernardo BC, Ooi JYY, Patterson NL, McMullen JR. Weeks KL, et al. Adv Exp Med Biol. 2017;1000:187-210. doi: 10.1007/978-981-10-4304-8_12. Adv Exp Med Biol. 2017. PMID: 29098623 Review. - The protective effects of exercise and phosphoinositide 3-kinase (p110alpha) in the failing heart.
Owen KL, Pretorius L, McMullen JR. Owen KL, et al. Clin Sci (Lond). 2009 Mar;116(5):365-75. doi: 10.1042/CS20080183. Clin Sci (Lond). 2009. PMID: 19175355 Review.
Cited by
- Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise.
Schüttler D, Clauss S, Weckbach LT, Brunner S. Schüttler D, et al. Cells. 2019 Sep 23;8(10):1128. doi: 10.3390/cells8101128. Cells. 2019. PMID: 31547508 Free PMC article. Review. - Interrelation between α-Cardiac Actin Treadmilling and Myocardin-Related Transcription Factor-A Nuclear Shuttling in Cardiomyocytes.
Gorey MA, Mericskay M, Li Z, Decaux JF. Gorey MA, et al. Int J Mol Sci. 2022 Jul 2;23(13):7394. doi: 10.3390/ijms23137394. Int J Mol Sci. 2022. PMID: 35806398 Free PMC article. - Reduced cardiac remodeling and function in cardiac-specific EP4 receptor knockout mice with myocardial infarction.
Qian JY, Harding P, Liu Y, Shesely E, Yang XP, LaPointe MC. Qian JY, et al. Hypertension. 2008 Feb;51(2):560-6. doi: 10.1161/HYPERTENSIONAHA.107.102590. Epub 2008 Jan 7. Hypertension. 2008. PMID: 18180401 Free PMC article. - Phosphoinositide-3 kinase signaling in cardiac hypertrophy and heart failure.
Aoyagi T, Matsui T. Aoyagi T, et al. Curr Pharm Des. 2011;17(18):1818-24. doi: 10.2174/138161211796390976. Curr Pharm Des. 2011. PMID: 21631421 Free PMC article. Review. - Ibrutinib impairs IGF-1-dependent activation of intracellular Ca handling in isolated mouse ventricular myocytes.
Tarnowski D, Feder AL, Trum M, Kreitmeier KG, Stengel L, Maier LS, Sag CM. Tarnowski D, et al. Front Cardiovasc Med. 2023 Aug 15;10:1190099. doi: 10.3389/fcvm.2023.1190099. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37655217 Free PMC article.
References
- Briazgounov IP. World Health Stat Q. 1988;41:242–250. - PubMed
- Barker WH, Mullooly JP, Getchell W. Circulation. 2006;113:799–805. - PubMed
- Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, et al. Circulation. 1997;95:766–770. - PubMed
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. N Engl J Med. 1990;322:1561–1566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 HD001487/HD/NICHD NIH HHS/United States
- R01 HL065742/HL/NHLBI NIH HHS/United States
- K12-HD001487/HD/NICHD NIH HHS/United States
- R01 HL65742/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases