Two regions of the tail are necessary for the isoform-specific functions of nonmuscle myosin IIB - PubMed (original) (raw)
Two regions of the tail are necessary for the isoform-specific functions of nonmuscle myosin IIB
Masaaki K Sato et al. Mol Biol Cell. 2007 Mar.
Abstract
To function in the cell, nonmuscle myosin II molecules assemble into filaments through their C-terminal tails. Because myosin II isoforms most likely assemble into homo-filaments in vivo, it seems that some self-recognition mechanisms of individual myosin II isoforms should exist. Exogenous expression of myosin IIB rod fragment is thus expected to prevent the function of myosin IIB specifically. We expected to reveal some self-recognition sites of myosin IIB from the phenotype by expressing appropriate myosin IIB rod fragments. We expressed the C-terminal 305-residue rod fragment of the myosin IIB heavy chain (BRF305) in MRC-5 SV1 TG1 cells. As a result, unstable morphology was observed like MHC-IIB(-/-) fibroblasts. This phenotype was not observed in cells expressing BRF305 mutants: 1) with a defect in assembling, 2) lacking N-terminal 57 residues (N-57), or 3) lacking C-terminal 63 residues (C-63). A myosin IIA rod fragment ARF296 corresponding to BRF305 was not effective. However, the chimeric ARF296, in which the N-57 and C-63 of BRF305 were substituted for the corresponding regions of ARF296, acquired the ability to induce unstable morphology. We propose that the N-57 and C-63 of BRF305 are involved in self-recognition when myosin IIB molecules assemble into homo-filament.
Figures
Figure 1.
Schematic diagrams of myosin II rod fragments and a full-length myosin II. The myosin II rod fragments were expressed as N-terminal GFP-fused proteins. Numbers indicate amino acid residues of MHC-IIA (for ARF296) and MHC-IIB (for other fragments). Dark and light grays represent the portions derived from myosin IIB and myosin IIA, respectively.
Figure 2.
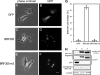
Expression of GFP-BRF305, but not its assembly-deficient mutant (GFP-BRF305-m3) in MRC-5 SV1 TG1 cells induced aberrant cell shape. The images were taken from live cells expressing GFP as control (A and B), GFP-BRF305 (C and D), and GFP-BRF305-m3 (E and F). Note that GFP signals were observed throughout the cytoplasm besides bright aggregates in the case of expression of GFP-fused rod fragments (D and F). Bar, 20 μm. (G) Percentage of cells with aberrant shapes seen 24 h after transfection were calculated from at least 70 cells expressing GFP fluorescence. Cells showing shrunken cell body with long protrusions or projecting multiple protrusions were regarded as “aberrant cell shape.” Error bars, ±SD of three independent experiments. (H) Immunoblot analyses for checking the expression levels of each exogenous protein. Lysates from the cells expressing GFP (lane 1), GFP-BRF305-m3 (lane 2), and GFP-BRF305 (lane 3) were analyzed by anti-MHC-IIB (C-term) pAb (top and forth panels), anti-GFP mAb (second and third panels), and anti-α-tubulin mAb (bottom panel). The signals of GFP-rod fragments (top panel) and endogenous MHC-IIB (forth panel) were detected simultaneously on the same immunoblot. α-tubulin was used as a loading control.
Figure 3.
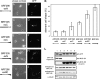
Aberrant cell shape was not induced by the expression of ARF296 or the deletion fragments of BRF305. The images were taken from live cells expressing GFP-ARF296 (A and B), GFP-BRF248 (C and D), and GFP-BRFΔC63 (E and F). Bar, 20 μm. (G) Percentage of cells with aberrant shapes seen 24 h after transfection were calculated from at least 70 cells expressing GFP fluorescence. Error bars, ±SD of three independent experiments. The effect of GFP-BRF305 was reconfirmed in each experiment. (H) Immunoblot analyses for checking the expression levels of each exogenous protein. Lysates from the cells expressing GFP-ARF296 (lane 1), GFP-BRF248 (lane 2), GFP-BRF305ΔC63 (lane 3), and GFP-BRF305 (lane 4) were analyzed using anti-GFP mAb (top panel), anti-MHC-IIA pAb (second and forth panels), anti-MHC-IIB (C-term) pAb (third and fifth panels), and anti-α-tubulin mAb (bottom panel). Because GFP-BRF305ΔC63 lacks a C-terminus, it was not detected by anti-MHC-IIB (C-term) pAb. The signals of GFP-rod fragments (second panel) and endogenous MHC-IIA (forth panel) were detected simultaneously on the same immunoblot. The signals of GFP-rod fragments (third panel) and endogenous MHC-IIB (fifth panel) were detected simultaneously on the same immunoblot. α-tubulin was used as a loading control.
Figure 4.
Induction of aberrant cell shape by the expression of BRF305-ARF296 chimeric fragments. The images were taken from live cells expressing GFP-ARF296exN (A and B), GFP-BRF305exC (C and D), GFP-ARF296exC (E and F), GFP-BRF305exN (G and H), and GFP-ARF296exNC (I and J). Bar, 20 μm. (K) Percentage of cells with aberrant shapes seen 24 h after transfection were calculated from at least 70 cells expressing GFP fluorescence. Error bars, ±SD of three independent experiments. The morphological scoring of cells was performed in a double-blind manner. The effect of GFP-BRF305 was reconfirmed in each experiment. (L) Immunoblot analyses for checking the expression levels of each exogenous protein. Lysates from the cells expressing GFP-ARF296exN (lane 1), GFP-BRF305exC (lane 2), GFP-ARF296exC (lane 3), GFP-BRF305exN (lane 4), GFP-ARF296exNC (lane 5), and GFP-BRF305 (lane 6) were analyzed by anti-GFP mAb (top panel), anti-MHC-IIA pAb (second and forth panels), anti-MHC-IIB (C-term) pAb (third and fifth panels), and anti-α-tubulin mAb (bottom panel). The signals of GFP-rod fragments (second panel) and endogenous MHC-IIA (forth panel) were detected simultaneously on the same immunoblot. The signals of GFP-rod fragments (third panel) and endogenous MHC-IIB (fifth panel) were detected simultaneously on the same immunoblot. α-tubulin was used as a loading control.
Figure 5.
Coimmunoprecipitation of BRF305 and ARF296exNC with endogenous myosin IIB. Lysates from the cells, each expressing GFP-ARF296, GFP-BRF305, GFP-ARF296exNC, and GFP-BRF305-m3 (indicated below the panels), were incubated with anti-MHC-IIB (N-term) pAb to immunoprecipitate endogenous myosin IIB. The immunocomplexes were collected and analyzed by immunoblotting using anti-MHC-IIB (C-term) pAb and anti-GFP mAb for detecting endogenous MHC-IIB and each GFP-rod fragments, respectively. Top and bottom panels indicate endogenous MHC-IIB and GFP-rod fragments, respectively. Left lanes (1, 4, 7, and 10) of each panel are total lysates before immunoprecipitation. Center lanes (2, 5, 8, and 11) are samples of the immunocomplex. Right lanes (3, 6, 9, and 12) are samples without antibodies as negative control of immunoprecipitation.
Figure 6.
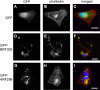
Disappearance of cortical actin fibers in GFP-BRF305–expressing cells, but not in GFP-ARF296–expressing cells. The cells expressing GFP as control (A–C), GFP-BRF305 (D–F), and GFP-ARF296 (G–I) were fixed and stained with TRITC-phalloidin (B, E, and H). (C, F, and I) Merged images of A and B, D and E, and G and H with DAPI staining (blue), respectively. The green and red colors indicate GFP and F-actin, respectively. Bar, 20 μm.
Figure 7.
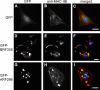
Myosin IIB remained at the cell cortex in GFP-ARF296–expressing cells. The cells expressing GFP as control (A–C), GFP-BRF305 (D–F), and GFP-ARF296 (G–I) were fixed and stained with anti-MHC-IIB pAb followed by detection with Cy3-labeled anti-rabbit IgG antibody (B, E, and H). Anti-MHC-IIB (C-term) pAb were used for GFP- and GFP-ARF296–transfected cells (B and H). Anti-MHC-IIB (N-term) pAb was used for GFP-BRF305 transfected cells (E) to distinguish between BRF305 and endogenous myosin IIB. (C, F, and I) Merged images of A and B, D and E, and G and H with DAPI staining (blue), respectively. The green and red colors indicate GFP and endogenous myosin IIB, respectively. Bar, 20 μm.
Figure 8.
Alignment of the amino acid sequences of N-57 and C-63 of BRF305 with corresponding region of ARF296. Schematic illustration represents the portions of N-57 and C-63 region in the rod fragment. (A) N-57 of BRF305 and corresponding ARF296. (B) C-63 of BRF305 and corresponding ARF296. Asterisks indicate identical amino acid residues.
Similar articles
- Dynamic assembly properties of nonmuscle myosin II isoforms revealed by combination of fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy.
Mitsuhashi M, Sakata H, Kinjo M, Yazawa M, Takahashi M. Mitsuhashi M, et al. J Biochem. 2011 Mar;149(3):253-63. doi: 10.1093/jb/mvq134. Epub 2010 Nov 23. J Biochem. 2011. PMID: 21106542 - Mts1 regulates the assembly of nonmuscle myosin-IIA.
Li ZH, Spektor A, Varlamova O, Bresnick AR. Li ZH, et al. Biochemistry. 2003 Dec 9;42(48):14258-66. doi: 10.1021/bi0354379. Biochemistry. 2003. PMID: 14640694 - Multiple S100 protein isoforms and C-terminal phosphorylation contribute to the paralog-selective regulation of nonmuscle myosin 2 filaments.
Ecsédi P, Billington N, Pálfy G, Gógl G, Kiss B, Bulyáki É, Bodor A, Sellers JR, Nyitray L. Ecsédi P, et al. J Biol Chem. 2018 Sep 21;293(38):14850-14867. doi: 10.1074/jbc.RA118.004277. Epub 2018 Aug 7. J Biol Chem. 2018. PMID: 30087119 Free PMC article. - Myosin II isoforms in smooth muscle: heterogeneity and function.
Eddinger TJ, Meer DP. Eddinger TJ, et al. Am J Physiol Cell Physiol. 2007 Aug;293(2):C493-508. doi: 10.1152/ajpcell.00131.2007. Epub 2007 May 2. Am J Physiol Cell Physiol. 2007. PMID: 17475667 Review. - Distinct and redundant roles of the non-muscle myosin II isoforms and functional domains.
Wang A, Ma X, Conti MA, Adelstein RS. Wang A, et al. Biochem Soc Trans. 2011 Oct;39(5):1131-5. doi: 10.1042/BST0391131. Biochem Soc Trans. 2011. PMID: 21936777 Free PMC article. Review.
Cited by
- Germinal center-specific protein human germinal center associated lymphoma directly interacts with both myosin and actin and increases the binding of myosin to actin.
Lu X, Kazmierczak K, Jiang X, Jones M, Watt J, Helfman DM, Moore JR, Szczesna-Cordary D, Lossos IS. Lu X, et al. FEBS J. 2011 Jun;278(11):1922-31. doi: 10.1111/j.1742-4658.2011.08109.x. Epub 2011 Apr 20. FEBS J. 2011. PMID: 21447067 Free PMC article. - MHC-IIB filament assembly and cellular localization are governed by the rod net charge.
Rosenberg M, Straussman R, Ben-Ya'acov A, Ronen D, Ravid S. Rosenberg M, et al. PLoS One. 2008 Jan 30;3(1):e1496. doi: 10.1371/journal.pone.0001496. PLoS One. 2008. PMID: 18231583 Free PMC article. - Myosin II tailpiece determines its paracrystal structure, filament assembly properties, and cellular localization.
Ronen D, Ravid S. Ronen D, et al. J Biol Chem. 2009 Sep 11;284(37):24948-57. doi: 10.1074/jbc.M109.023754. Epub 2009 Jun 24. J Biol Chem. 2009. PMID: 19553683 Free PMC article. - High resolution characterization of myosin IIC protein tailpiece and its effect on filament assembly.
Rosenberg MM, Ronen D, Lahav N, Nazirov E, Ravid S, Friedler A. Rosenberg MM, et al. J Biol Chem. 2013 Apr 5;288(14):9779-9789. doi: 10.1074/jbc.M112.430173. Epub 2013 Feb 20. J Biol Chem. 2013. PMID: 23426373 Free PMC article. - Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1.
Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, Minowa A, Akashi H, Arase H, Kawaoka Y, Kawaguchi Y. Arii J, et al. Nature. 2010 Oct 14;467(7317):859-62. doi: 10.1038/nature09420. Nature. 2010. PMID: 20944748
References
- Bao J., Jana S. S., Adelstein R. S. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J. Biol. Chem. 2005;280:19594–19599. - PubMed
- Ben-Ya'acov A., Ravid S. Epidermal growth factor-mediated transient phosphorylation and membrane localization of myosin II-B are required for efficient chemotaxis. J. Biol. Chem. 2003;278:40032–40040. - PubMed
- Betapudi V., Licate L. S., Egelhoff T. T. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–4733. - PubMed
- Brown M. E., Bridgman P. C. Retrograde flow rate is increased in growth cones from myosin IIB knockout mice. J. Cell Sci. 2003;116:1087–1094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials