The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection - PubMed (original) (raw)
The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection
Zhao Zhong Chong et al. Int J Mol Med. 2007 Feb.
Abstract
Microglia of the central nervous system serve a variety of functions that may ultimately lead to the development or detriment of neighboring neuronal and vascular cells. These scavengers of the nervous system have been associated with a variety of neurodegenerative disorders, but the toxic potential of microglia is equally balanced by the protective nature of these cells to exclude foreign microorganisms and promote new tissue proliferation and reorganization. To this extent, our work outlines a series of endogenous microglial cellular pathways that can constitute protection for microglia against during oxygen-glucose deprivation (OGD). We demonstrate in both primary microglia and the microglial cell line EOC 2 that endogenous microglial protection against OGD relies upon the activation and expression of the phosphatidylinositol 3-kinase pathways of mammalian target of rapamycin (mTOR) and protein kinase B (Akt1), since pharmacological inhibition of mTOR or Akt1 as well as the gene silencing of Akt1 protein expression leads to significantly increased microglial apoptotic cell injury, DNA fragmentation, and membrane phosphatidylserine exposure. The mTOR pathway may offer endogenous protection through mechanisms that do not entirely rely upon inhibition of glycogen synthase kinase-3beta (GSK-3beta) activity while Akt1 appears to converge upon the necessary blockade of GSK-3beta. Closely aligned to these endogenous protective mechanisms is the subcellular presence and nuclear translocation of nuclear factor-kappaB p65 (NF-kappaB p65), since microglial cell injury is significantly increased during the gene silencing of NF-kappaB p65. Elucidating the underlying pathways that can afford endogenous protection and maintain functional integrity of microglia should offer new prospects for the treatment of a broad range of nervous system disorders.
Figures
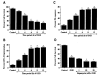
Figure 1
OGD leads to progressive primary microglial cell apoptotic injury that is reduced by mTOR activation. (A), Primary microglia were exposed to OGD for 2, 4, 6, 8, and 12 h and cell survival was determined 24 h after OGD. The cell survival was progressively decreased over a period of 2, 4, 6, 8, and 12 h of OGD (*P<0.01 vs. untreated control). (B), Primary microglia were exposed to OGD for 2, 4, 6, 8, and 12 h and DNA fragmentation with TUNEL was determined 24 h after OGD. Apoptotic DNA fragmentation was progressively increased over a period of 2, 4, 6, 8, and 12 h of OGD (*P<0.01 vs. untreated control). (C), Primary microglia were exposed to OGD for 2, 4, 6, 8, and 12 h and membrane PS exposure with Annexin-V was determined 24 h after OGD. Early apoptotic PS externalization was progressively increased over a period of 2, 4, 6, 8, and 12 h of OGD (*P<0.01 vs. untreated control). (D), The mTOR inhibitor rapamycin at concentrations of 1.0-20.0 nM was applied to the EOC 2 microglial cell line 1 h prior to a 6-h period of OGD and cell survival was assessed 24 h later. Increased microglial cell injury by rapamycin administration during OGD was evident in microglial when compared with cultures exposed to OGD alone (*P<0.01 vs. control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM. Control indicates untreated cultures.
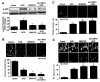
Figure 2
Akt1 confers endogenous protection to microglial during OGD. (A), Equal amounts of primary microglial protein extracts (50 µg/lane) were immunoblotted with anti-phospho-Akt1 (p-Akt1, active form) antibody 12 h following a 6-h period of OGD exposure. Application of the specific Akt1 inhibitor SH6 (20 µM) or transient transfection of Akt1 siRNA in primary microglia for 72 h prior to OGD blocked the expression of p-Akt1 (*P<0.01 vs. control; †P<0.01 vs. OGD). (B), Representative images and quantification of data are illustrated for trypan blue staining in primary microglial cells 24 h following a 6-h period of OGD. SH6 (20 µM) application or transfection with Akt1 siRNA significantly decreased cell survival during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). Green arrows indicate trypan blue uptake into microglia while white arrows illustrate absence of trypan blue uptake. (C), Representative images and quantification of data are illustrated for DNA fragmentation with TUNEL in primary microglial cells 24 h following a 6-h period of OGD. SH6 (20 µM) application or transfection with Akt1 siRNA for 72 h prior to OGD significantly increased DNA fragmentation during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). Green arrows indicate DNA fragmentation in microglia while white arrows illustrate absence of genomic DNA injury. (D), Representative images and quantification of data are illustrated for early apoptotic PS exposure with Annexin-V in primary microglial cells 24 h following a 6-h period of OGD. SH6 (20 µM) application or transfection with Akt1 siRNA for 72 h prior to OGD significantly increased membrane PS externalization during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM. Control indicates untreated cultures.
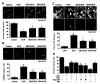
Figure 3
Inhibition of glycogen synthase kinase-3β (GSK-3β) activity enhances microglia integrity during OGD. The GSK-3β inhibitors SB216763 (SB21, 5 µM) or SB415286 (SB41, 25 µM) were applied to primary microglia 1 h prior to a 6-h period of OGD. Cell survival, DNA fragmentation, and membrane PS exposure were determined 24 h following OGD using the trypan blue dye exclusion method, TUNEL assay, or Annexin-V labeling respectively. (A), Representative images and quantification of data illustrate trypan blue staining in microglia following OGD. Application of SB216763 (SB21, 5 µM) or SB415286 (SB41, 25 µM) significantly decreased cell staining and increased microglial survival during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). Green arrows indicate trypan blue uptake into microglia while white arrows illustrate absence of trypan blue uptake. (B), Representative images and quantification of data illustrate DNA fragmentation with TUNEL in microglia following OGD. Application of SB216763 (SB21, 5 µM) or SB415286 (SB41, 25 µM) significantly decreased TUNEL staining and decreased microglial DNA fragmentation during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). Green arrows indicate DNA fragmentation in microglia while white arrows illustrate absence of genomic DNA injury. (C), Representative images and quantification of data illustrate membrane PS exposure with Annexin-V in microglia following OGD. Application of SB216763 (SB21, 5 µM) or SB415286 (SB41, 25 µM) significantly decreased Annexin-V staining and decreased microglial PS externalization during OGD (*P<0.01 vs. control; †P<0.01 vs. OGD). (D), The mTOR inhibitor rapamycin (Rapa) at concentrations of 1.0-20.0 nM in conjunction with SB216763 (SB21, 5 µM) or SH6 (20 µM) was applied to the EOC 2 microglial cell line 1 h prior to a 6-h period of OGD and cell survival was assessed 24 h later. Concurrent inhibition of mTOR activity with rapamycin during SB21 administration reduced the beneficial effects of GSK-3β inhibition and worsened microglial survival to levels slightly greater than during OGD alone. In contrast, concurrent inhibition of Akt1 activity did not alter protection during GSK-3β inhibition or lead to a synergistic benefit (*P<0.01 vs. OGD; †P<0.01 vs. OGD/SB21). In all cases, each data point represents the mean and SEM. Control or CON indicates untreated cultures.
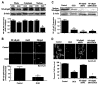
Figure 4
OGD prevents the nuclear translocation of NF-κB in microglia and gene silencing of NF-κB enhances microglial cell injury. (A), Equal amounts of protein extracts (50 µg/lane) from whole cells, the cytoplasm, and the nucleus were immunoblotted with anti-NF-κB p65 antibody 6 h following a 6-h period of OGD. Expression of NF-κB p65 was significantly diminished in whole and nuclear fractions during OGD exposure (*P<0.01 vs. CON). CON, untreated cultures. (B), Subcellular location of NF-κB p65 was followed at 6 h following OGD with immunofluorescent staining with anti-NF-κB p65 antibody and visualized with Texas-Red streptavidin. Microglial cell nuclei were stained with DAPI. In merged images, cells with OGD with green arrows show neuronal nuclei with strong NF-κB p65 staining (pink) and white arrows show neuronal cytoplasm with decreased NF-κB p65 staining (purple), illustrating OGD prevent nuclear translocation of NF-κB p65 in primary microglia. Control, untreated microglia. (C), Equal amounts of microglial protein extracts (50 µg/lane) were immunoblotted with anti-NF-κB p65 antibody 6 h following a 6-h period of OGD exposure. Transfection of NF-κB p65 siRNA in microglia for 72 h significantly blocked the expression of NF-κB p65 in control cell and during OGD (*P<0.01 vs. control). (D), Transient transfection of NF-κB p65 siRNA in primary microglia for 72 h prior to OGD was performed and cell survival was determined 24 h following OGD using trypan blue dye exclusion method. Transfection of NF-κB p65 siRNA in microglia prior to a 6-h period of OGD resulted in increased cell injury when compared to OGD alone (*P<0.01 vs. control; †P<0.01 vs. OGD). NF-κB p65 siRNA alone was not toxic to cells. Green arrows indicate trypan blue uptake into microglia while white arrows illustrate absence of trypan blue uptake. In all cases, each data point represents the mean and SEM. Control, untreated control cultures.
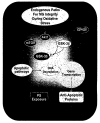
Figure 5
Endogenous microglial protection is conveyed through a series of cellular pathways that rely upon the regulation of mTOR, Akt1, GSK-3β, and NF-κB. During oxidative stress, endogenous protection in microglial cells (MG) requires the activation of Akt1 and the mTOR pathways. The activation of these cellular mechanisms, especially those involving Akt1, can lead to the inhibitory phosphorylation (p) of GSK-3β to block early and late apoptotic pathways that involve genomic DNA degradation and membrane PS exposure. In addition, GSK-3β activity may be linked to NF-κB gene transcription, but it is the maintenance of NF-κB expression with its subsequent nuclear translocation that can offer an additional level of endogenous protection in microglial cells during oxidative stress to promote anti-apoptotic pathways.
Similar articles
- Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB.
Li F, Chong ZZ, Maiese K. Li F, et al. Curr Neurovasc Res. 2006 Aug;3(3):187-201. doi: 10.2174/156720206778018758. Curr Neurovasc Res. 2006. PMID: 16918383 Free PMC article. - Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways.
Chong ZZ, Li F, Maiese K. Chong ZZ, et al. Cell Signal. 2007 Jun;19(6):1150-62. doi: 10.1016/j.cellsig.2006.12.009. Epub 2007 Jan 9. Cell Signal. 2007. PMID: 17289346 Free PMC article. - Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades.
Wang MJ, Huang HY, Chen WF, Chang HF, Kuo JS. Wang MJ, et al. J Neuroinflammation. 2010 Dec 31;7:99. doi: 10.1186/1742-2094-7-99. J Neuroinflammation. 2010. PMID: 21194439 Free PMC article. - Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system.
Chong ZZ, Maiese K. Chong ZZ, et al. Histol Histopathol. 2004 Apr;19(2):495-504. doi: 10.14670/HH-19.495. Histol Histopathol. 2004. PMID: 15024710 Free PMC article. Review. - Glycogen synthase kinase 3beta as a target for the therapy of shock and inflammation.
Dugo L, Collin M, Thiemermann C. Dugo L, et al. Shock. 2007 Feb;27(2):113-23. doi: 10.1097/01.shk.0000238059.23837.68. Shock. 2007. PMID: 17224784 Review.
Cited by
- Targeting cardiovascular disease with novel SIRT1 pathways.
Chong ZZ, Wang S, Shang YC, Maiese K. Chong ZZ, et al. Future Cardiol. 2012 Jan;8(1):89-100. doi: 10.2217/fca.11.76. Future Cardiol. 2012. PMID: 22185448 Free PMC article. Review. - Mammalian target of rapamycin signaling in diabetic cardiovascular disease.
Chong ZZ, Maiese K. Chong ZZ, et al. Cardiovasc Diabetol. 2012 Jul 16;11:45. doi: 10.1186/1475-2840-11-45. Cardiovasc Diabetol. 2012. PMID: 22545721 Free PMC article. Review. - Raves and risks for erythropoietin.
Maiese K, Chong ZZ, Shang YC. Maiese K, et al. Cytokine Growth Factor Rev. 2008 Apr;19(2):145-55. doi: 10.1016/j.cytogfr.2008.01.004. Epub 2008 Mar 4. Cytokine Growth Factor Rev. 2008. PMID: 18299246 Free PMC article. Review. - Sexual dimorphism in the white matter of rodents.
Cerghet M, Skoff RP, Swamydas M, Bessert D. Cerghet M, et al. J Neurol Sci. 2009 Nov 15;286(1-2):76-80. doi: 10.1016/j.jns.2009.06.039. Epub 2009 Jul 22. J Neurol Sci. 2009. PMID: 19625027 Free PMC article. Review. - The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury.
Chong ZZ, Maiese K. Chong ZZ, et al. Histol Histopathol. 2007 Nov;22(11):1251-67. doi: 10.14670/HH-22.1251. Histol Histopathol. 2007. PMID: 17647198 Free PMC article. Review.
References
- Di Rosa M, Del'Ombra N, Zambito AM, Malaguarnera M, Nicoletti F, Malaguarnera L. Chitotriosidase and inflammatory mediator levels in Alzheimer's disease and cerebrovascular dementia. Eur J Neurosci. 2006;23:2648–2656. - PubMed
- Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res. 2005;2:409–423. - PubMed
- Tabet N. Acetylcholinesterase inhibitors for Alzheimer's disease: anti-inflammatories in acetylcholine clothing! Age Ageing. 2006;35:336–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES006639/ES/NIEHS NIH HHS/United States
- R01 NS053946/NS/NINDS NIH HHS/United States
- R01 NS053946-01A2/NS/NINDS NIH HHS/United States
- P30 ES 06639/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous