Allosteric activation of the extracellular Ca2+-sensing receptor by L-amino acids enhances ERK1/2 phosphorylation - PubMed (original) (raw)
Allosteric activation of the extracellular Ca2+-sensing receptor by L-amino acids enhances ERK1/2 phosphorylation
Heather J Lee et al. Biochem J. 2007.
Abstract
The calcium-sensing receptor (CaR) mediates feedback control of Ca2+o (extracellular Ca2+) concentration. Although the mechanisms are not fully understood, the CaR couples to several important intracellular signalling enzymes, including PI-PLC (phosphoinositide-specific phospholipase C), leading to Ca2+i (intracellular Ca2+) mobilization, and ERK1/2 (extracellular-signal-regulated kinase 1/2). In addition to Ca2+o, the CaR is activated allosterically by several subclasses of L-amino acids, including the aromatics L-phenylalanine and L-tryptophan. These amino acids enhance the Ca2+o-sensitivity of Ca2+i mobilization in CaR-expressing HEK-293 (human embryonic kidney) cells and normal human parathyroid cells. Furthermore, on a background of a physiological fasting serum L-amino acid mixture, they induce a small, but physiologically significant, enhancement of Ca2+o-dependent suppression of PTH (parathyroid hormone) secretion. The impact of amino acids on CaR-stimulated ERK1/2, however, has not been determined. In the present study, we examined the effects of L-amino acids on Ca2+o-stimulated ERK1/2 phosphorylation as determined by Western blotting and a newly developed quantitative assay (SureFire). L-Amino acids induced a small, but significant, enhancement of Ca2+o-stimulated ERK1/2. In CaR-expressing HEK-293 cells, 10 mM L-phenylalanine lowered the EC50 for Ca2+o from approx. 2.3 to 2.0 mM in the Western blot assay and from 3.4 to 2.9 mM in the SureFire assay. The effect was stereoselective (L>D), and another aromatic amino acid, L-tryptophan, was also effective. The effects of amino acids were investigated further in HEK-293 cells that expressed the CaR mutant S169T. L-Phenylalanine normalized the EC50 for Ca2+o-stimulated Ca2+i mobilization from approx. 12 mM to 5.0 mM and ERK1/2 phosphorylation from approx. 4.6 mM to 2.6 mM. Taken together, the data indicate that L-phenylalanine and other amino acids enhance the Ca2+o-sensitivity of CaR-stimulated ERK1/2 phosphorylation; however, the effect is comparatively small and operates in the form of a fine-tuning mechanism.
Figures
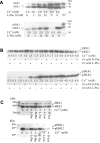
Figure 1. Activation of Ca2+o-stimulated ERK1/2 by calcimimetic NPS R467 in CaR-expressing HEK-293 cells
HEK-293 cells that stably expressed the human wild-type CaR were incubated in PSS that contained various Ca2+o concentrations in the absence or presence of 1 μM NPS R467. The incubations were stopped by the addition of ice-cold PBS and the cells were lysed. In (A) and (B), the samples were processed by SDS/PAGE and Western blotting, and the blots were developed for total and phosphorylated ERK1/2 and then quantified as described in the Experimental section. The data were then expressed as a percentage of the maximum level of control phosphorylation for each experiment. (A) A representative Western blot for total (upper panel) and phosphorylated (lower panel) ERK1/2. Molecular masses are indicated in kDa. (B) Ca2+o concentration–response data showing the effect of 1 μM NPS R467 (R-467) on Ca2+o-stimulated ERK1/2 phosphorylation detected by Western blotting. In (C), the samples were processed for SureFire cellular ERK1/2 analysis as described in the Experimental section. ○, Control; ▲, NPS R467.
Figure 2. Effects of L-phenylalanine on Ca2+o-stimulated ERK1/2 phosphorylation in CaR-expressing HEK-293 cells
CaR-expressing HEK-293 cells were incubated in the presence of 2 mM Ca2+ in the absence (−) or presence (+) of 10 mM
L
-phenylalanine for 10 min. The incubations were then stopped by exposure to ice-cold PBS, and the cells were lysed and processed by SDS/PAGE and Western blotting. Films were developed by ECL® as described in the Experimental section.
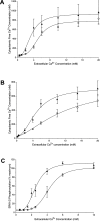
Figure 3. Effects of L-phenylalanine on the Ca2+o-sensitivity of ERK1/2 phosphorylation in CaR-expressing HEK-293 cells
Wild-type CaR-expressing HEK-293 cells were incubated (A and B) in the presence of various Ca2+ concentrations (0.5–6.0 mM) in the absence or presence of 10 mM
L
-phenylalanine for 10 min. The incubations were stopped by exposure to ice-cold PBS, and the cells were then lysed and processed by SDS/PAGE and Western blotting as described in the Experimental section. All Ca2+o-concentration-dependent data were expressed as a percentage of the maximum level of phosphorylation obtained for each control data set. (A) Representative blot demonstrating a small enhancement of the Ca2+-sensitivity of ERK1/2 phosphorylation by 10 mM
L
-phenylalanine in the region of the EC50 for Ca2+o (approx. 2.0 mM). (B) Ca2+-concentration-dependent activation of ERK1/2 phosphorylation in the absence or presence of 10 mM
L
-phenylalanine from a total of 25 experiments. (C) CaR-expressing HEK-293 cells were incubated in the presence of various Ca2+o concentrations between 0.5 and 10 mM and then processed for the SureFire ERK1/2 assay as described in the Experimental section (_n_=14). ○, Control; ▲, 10 mM
L
-phenylalanine.
Figure 4. Concentration-dependence and stereoselectivity of L-phenylalanine and effects of various other amino acids
HEK-293 cells that stably expressed the wild-type CaR were pre-incubated for 30 min and then exposed to (A) various concentrations of Ca2+ (0.5, 2.0 and 6.0 mM) and
L
-phenylalanine (0–30 mM) as shown, (B) various concentrations of Ca2+ in the presence of 10 mM
L
-phenylalanine or
D
-phenylalanine and (C) various concentrations of Ca2+ (0.5, 2.0 and 6.0 mM) in the absence or presence of various amino acids as shown. The samples were processed by SDS/PAGE and Western blotting as described in the Experimental section. In (A), lanes between the
L
-phenylalanine concentration–response data (lanes 1–4) and low- and high- Ca2+ controls (shown as lanes 5 and 6 from the left) have been deleted from the image. Molecular masses are indicated in kDa.
Figure 5. Comparison of surface expression between the wild-type CaR and CaR mutant S169T
HEK-293 cells were transiently transfected with FLAG-tagged wild-type or S169T mutant CaRs as described in the Experimental section. The cells were then labelled with sulfo-_N_-hydroxysuccinimido-biotin and lysed in the presence of 100 mM iodoacetamide. CaR proteins were immunoprecipitated using anti-FLAG antibody, suspended in SDS/PAGE sample buffer and assayed for protein content. Equivalent amounts of protein were then processed by SDS/PAGE. Western blotting and detection of biotin-labelled proteins was performed using avidin–HRP and ECL® as described in the Experimental section. The arrows indicate the molecular masses of markers in kDa. The lower-molecular-mass form (approx. 160 kDa) is likely to represent monomers of the mature CaR [32]. The higher-molecular-mass species is presumed to correspond to homodimers.
Figure 6. Effects of L-phenylalanine on Ca2+i mobilization in HEK-293 cells expressing the wild-type CaR and on Ca2+i mobilization and ERK1/2 phosphorylation in HEK-293 cells expressing S169T
HEK-293 cells that stably expressed the wild-type CaR (A) or S169T mutant CaR (B) were loaded with fura 2 and investigated for Ca2+o- and
L
-phenylalanine-stimulated Ca2+i mobilization by microfluorimetry. In (A) and (B), the receptor response is expressed in the form of a calibrated cytoplasmic free Ca2+ concentration (in nM). Compared with the wild-type CaR, S169T exhibited markedly impaired sensitivity to Ca2+o that was restored to near-normal Ca2+o-sensitivity by the addition of 10 mM
L
-phenylalanine. (C) Ca2+o concentration–response data for the activation of ERK1/2 as determined by the SureFire assay in the absence and presence of
L
-phenylalanine in HEK-293 cells that stably expressed the S169T mutant. The data in (A) were obtained in nine experiments. The data in (B) and (C) were obtained in six experiments. ○, Control; ▲, 10 mM
L
-phenylalanine.
Similar articles
- Endogenous expression and protein kinase A-dependent phosphorylation of the guanine nucleotide exchange factor Ras-GRF1 in human embryonic kidney 293 cells.
Norum JH, Méthi T, Mattingly RR, Levy FO. Norum JH, et al. FEBS J. 2005 May;272(9):2304-16. doi: 10.1111/j.1742-4658.2005.04658.x. FEBS J. 2005. PMID: 15853814 - Involvement of protein kinase C-alpha and -epsilon in extracellular Ca(2+) signalling mediated by the calcium sensing receptor.
Sakwe AM, Larsson M, Rask L. Sakwe AM, et al. Exp Cell Res. 2004 Jul 15;297(2):560-73. doi: 10.1016/j.yexcr.2004.03.039. Exp Cell Res. 2004. PMID: 15212956 - Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells.
Hira T, Nakajima S, Eto Y, Hara H. Hira T, et al. FEBS J. 2008 Sep;275(18):4620-6. doi: 10.1111/j.1742-4658.2008.06604.x. Epub 2008 Aug 8. FEBS J. 2008. PMID: 18691347 - Agonists and allosteric modulators of the calcium-sensing receptor and their therapeutic applications.
Saidak Z, Brazier M, Kamel S, Mentaverri R. Saidak Z, et al. Mol Pharmacol. 2009 Dec;76(6):1131-44. doi: 10.1124/mol.109.058784. Epub 2009 Sep 24. Mol Pharmacol. 2009. PMID: 19779033 Review. - The calcium-sensing receptor (CaR) permits Ca2+ to function as a versatile extracellular first messenger.
Brown EM, Chattopadhyay N, Vassilev PM, Hebert SC. Brown EM, et al. Recent Prog Horm Res. 1998;53:257-80; discussion 280-1. Recent Prog Horm Res. 1998. PMID: 9769711 Review.
Cited by
- Allosteric modulation of the calcium-sensing receptor by gamma-glutamyl peptides: inhibition of PTH secretion, suppression of intracellular cAMP levels, and a common mechanism of action with L-amino acids.
Broadhead GK, Mun HC, Avlani VA, Jourdon O, Church WB, Christopoulos A, Delbridge L, Conigrave AD. Broadhead GK, et al. J Biol Chem. 2011 Mar 18;286(11):8786-97. doi: 10.1074/jbc.M110.149724. Epub 2010 Dec 27. J Biol Chem. 2011. PMID: 21187282 Free PMC article. - Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery.
Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A, Conn PJ, Lindsley CW. Melancon BJ, et al. J Med Chem. 2012 Feb 23;55(4):1445-64. doi: 10.1021/jm201139r. Epub 2012 Jan 6. J Med Chem. 2012. PMID: 22148748 Free PMC article. No abstract available. - International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function.
Leach K, Hannan FM, Josephs TM, Keller AN, Møller TC, Ward DT, Kallay E, Mason RS, Thakker RV, Riccardi D, Conigrave AD, Bräuner-Osborne H. Leach K, et al. Pharmacol Rev. 2020 Jul;72(3):558-604. doi: 10.1124/pr.119.018531. Pharmacol Rev. 2020. PMID: 32467152 Free PMC article. Review. - Calcium Sensing Receptor as a Novel Mediator of Adipose Tissue Dysfunction: Mechanisms and Potential Clinical Implications.
Bravo-Sagua R, Mattar P, Díaz X, Lavandero S, Cifuentes M. Bravo-Sagua R, et al. Front Physiol. 2016 Sep 8;7:395. doi: 10.3389/fphys.2016.00395. eCollection 2016. Front Physiol. 2016. PMID: 27660614 Free PMC article. Review. - Anti-inflammatory role of extracellular l-arginine through calcium sensing receptor in human renal proximal tubular epithelial (HK-2) cells.
Shin S, Awuah Boadi E, Shah S, Ezell M, Li P, Bandyopadhyay BC. Shin S, et al. Int Immunopharmacol. 2023 Apr;117:109853. doi: 10.1016/j.intimp.2023.109853. Epub 2023 Feb 22. Int Immunopharmacol. 2023. PMID: 36827919 Free PMC article.
References
- Conigrave A. D., Quinn S. J., Brown E. M. Cooperative multi-modal sensing and therapeutic implications of the extracellular Ca2+-sensing receptor. Trends Pharm. Sci. 2000;21:401–407. - PubMed
- Hofer A. M., Brown E. M. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003;4:530–538. - PubMed
- Breitwieser G. E., Miedlich S. U., Zhang M. Calcium sensing receptors as integrators of multiple metabolic signals. Cell Calcium. 2004;35:209–216. - PubMed
- Quinn S. J., Kifor O., Trivedi S., Diaz R., Vassilev P., Brown E. M. Sodium and ionic strength sensing by the calcium receptor. J. Biol. Chem. 1998;273:19579–19586. - PubMed
- Quinn S. J., Bai M., Brown E. M. pH sensing by the calcium-sensing receptor. J. Biol. Chem. 2004;279:37241–37249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous