Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex - PubMed (original) (raw)
Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex
Qinghong Zhang et al. Proc Natl Acad Sci U S A. 2007.
Retraction in
- Retraction for Zhang et al., Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex.
Zhang Q, Wang SY, Fleuriel C, Dominique L, Rocheleau JV, Piston DW, Goodman RH. Zhang Q, et al. Proc Natl Acad Sci U S A. 2015 Feb 17;112(7):E819. doi: 10.1073/pnas.1501052112. Epub 2015 Feb 2. Proc Natl Acad Sci U S A. 2015. PMID: 25646486 Free PMC article. No abstract available.
Abstract
The Sir2 histone deacetylases are important for gene regulation, metabolism, and longevity. A unique feature of these enzymes is their utilization of NAD(+) as a cosubstrate, which has led to the suggestion that Sir2 activity reflects the cellular energy state. We show that SIRT1, a mammalian Sir2 homologue, is also controlled at the transcriptional level through a mechanism that is specific for this isoform. Treatment with the glycolytic blocker 2-deoxyglucose (2-DG) decreases association of the redox sensor CtBP with HIC1, an inhibitor of SIRT1 transcription. We propose that the reduction in transcriptional repression mediated by HIC1, due to the decrease of CtBP binding, increases SIRT1 expression. This mechanism allows the specific regulation of SIRT1 in response to nutrient deprivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
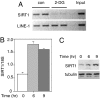
2-DG decreases free NADH levels and reduces CtBP binding to HIC1. (A) 2-DG changes the cellular lactate:pyruvate ratio. Primary human fibroblasts were treated with 10 mM 2-DG and the pyruvate and lactate concentrations were determined as described (15). (B) 2-DG decreases nuclear NAD(P)H levels. Two-photon fluorescence measurement of the nuclear NAD(P)H intensity of control and 2-DG-treated fibroblasts is shown. (C) 2-DG blocks the CtBP-HIC1 interaction. CtBP1 and FLAG-tagged HIC1 were transfected into Cos7 cells. Cells were treated with 2-DG before immunoprecipitation with anti-FLAG antibody. CtBP1 associated with HIC1 was assayed by Western blotting. con, control. (D) Interaction of endogenous CtBP and HIC1 is prevented by 2-DG treatment. Human primary fibroblasts were treated with 2-DG and immunoprecipitated with an antibody directed against CtBP. The associated HIC1 was assayed by Western blotting.
Fig. 2.
2-DG decreases CtBP recruitment to the SIRT1 promoter and increases SIRT1 expression. (A) CtBP recruitment to the SIRT1 promoter is blocked by 2-DG. Human primary fibroblasts were treated with 2-DG for 3 h, and ChIP was performed with antibodies directed against CtBP. Recruitment of CtBP to the HIC1-binding sites on the SIRT1 promoter was assayed by PCR. LINE-1 served as nonspecific control. (B) 2-DG increases SIRT1 transcript levels. Primary human fibroblasts were treated with 2-DG for 6 or 9 h, and the SIRT1 transcript was measured by RT-PCR. Data were normalized to 18S RNA and are represented as mean ± SEM from three independent experiments. (C) 2-DG increases SIRT1 protein levels. Primary human fibroblasts were treated with 2-DG for 6 or 9 h, and SIRT1 protein was assayed by Western blotting. Tubulin served as a loading control.
Fig. 3.
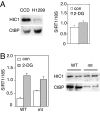
HIC1 is required for the transcriptional regulation of SIRT1 by 2-DG. (A) Decreased HIC1 levels in tumor cells are associated with loss of 2-DG-induced SIRT1 up-regulation. Human lung cancer cells (H1299) and primary human fibroblasts (CCD) were treated with 2-DG. Western blot (Left) shows the HIC1 and CtBP levels. SIRT1 RNA levels were measured by RT-PCR (Right). Data were normalized to 18S RNA and are represented as mean ± SEM from three independent experiments. (B) HIC1 rescues the 2-DG-induced SIRT1 up-regulation in tumor cells. H1299 lung cancer cells were transfected with HIC1, either WT or the GLDLSKK-deleted (mt) version, and treated with 2-DG. SIRT1 RNA was measured by RT-PCR and normalized to 18S RNA (Left). Data are represented as mean ± SEM from three independent experiments. Western blot shows the decreased binding of CtBP to the HIC1 GLDLSKK-deletion mutant compared with the WT (Right).
Fig. 4.
CtBP is required for the transcriptional regulation of SIRT1 by 2-DG. (A) Increased levels of SIRT1 transcript in CtBP-null MEFs. SIRT1 transcription in CtBP-null and heterozygous MEFs was measured by RT-PCR. Data were normalized to 18S RNA and are represented as mean ± SEM from three independent experiments. (B) CtBP knockdown in primary human fibroblasts up-regulates SIRT1 transcription. (Left) CtBP1 and -2 in human fibroblasts were knocked down by siRNA. (Right) Cells treated with siRNA (or control) were exposed to 2-DG before measurement of SIRT1 transcript levels. Data were normalized to 18S RNA and are represented as mean ± SEM from three independent experiments. con, control.
Fig. 5.
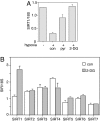
SIRT1 regulation by hypoxia and 2-DG. (A) Hypoxia decreases SIRT1 transcription. Primary human fibroblasts were treated overnight with hypoxia (1% O2) alone or in combination with 30 mM pyruvate (pyr) or 10 mM 2-DG. SIRT1 transcripts were measured by RT-PCR. Data were normalized to 18S RNA and are represented as mean ± SEM from three independent experiments. con, control. (B) 2-DG specifically increases SIRT1 transcript levels. Primary human fibroblasts were treated with 2-DG for 6 h, and SIRT1–7 transcripts were measured by RT-PCR. Data were normalized to 18S RNA and are represented as mean ± SEM from three independent experiments.
Fig. 6.
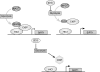
Models for CtBP-regulation of SIRT1 expression. (Upper) The reduction in free nuclear NADH after 2-DG treatment (designated by the smaller oval representing NADH) decreases CtBP association with HIC1, thus increasing SIRT1 transcription. (Lower) Posttranslational modification of CtBP induced by the block in glycolysis prevents association with HIC1.
Similar articles
- Differential regulation of HIC1 target genes by CtBP and NuRD, via an acetylation/SUMOylation switch, in quiescent versus proliferating cells.
Van Rechem C, Boulay G, Pinte S, Stankovic-Valentin N, Guérardel C, Leprince D. Van Rechem C, et al. Mol Cell Biol. 2010 Aug;30(16):4045-59. doi: 10.1128/MCB.00582-09. Epub 2010 Jun 14. Mol Cell Biol. 2010. PMID: 20547755 Free PMC article. - Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses.
Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Chen WY, et al. Cell. 2005 Nov 4;123(3):437-48. doi: 10.1016/j.cell.2005.08.011. Cell. 2005. PMID: 16269335 - SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress.
Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. Kobayashi Y, et al. Int J Mol Med. 2005 Aug;16(2):237-43. Int J Mol Med. 2005. PMID: 16012755 - Transcriptional regulation by C-terminal binding proteins.
Chinnadurai G. Chinnadurai G. Int J Biochem Cell Biol. 2007;39(9):1593-607. doi: 10.1016/j.biocel.2007.01.025. Epub 2007 Feb 4. Int J Biochem Cell Biol. 2007. PMID: 17336131 Review. - Transcriptional targets of sirtuins in the coordination of mammalian physiology.
Feige JN, Auwerx J. Feige JN, et al. Curr Opin Cell Biol. 2008 Jun;20(3):303-9. doi: 10.1016/j.ceb.2008.03.012. Epub 2008 May 28. Curr Opin Cell Biol. 2008. PMID: 18468877 Free PMC article. Review.
Cited by
- SIRT Is Required for EDP-Mediated Protective Responses toward Hypoxia-Reoxygenation Injury in Cardiac Cells.
Samokhvalov V, Jamieson KL, Fedotov I, Endo T, Seubert JM. Samokhvalov V, et al. Front Pharmacol. 2016 May 17;7:124. doi: 10.3389/fphar.2016.00124. eCollection 2016. Front Pharmacol. 2016. PMID: 27242531 Free PMC article. - Sirtuins and pyridine nucleotides.
Abdellatif M. Abdellatif M. Circ Res. 2012 Aug 17;111(5):642-56. doi: 10.1161/CIRCRESAHA.111.246546. Circ Res. 2012. PMID: 22904043 Free PMC article. Review. - Hypermethylation of the HIC1 promoter and aberrant expression of HIC1/SIRT1 contribute to the development of thyroid papillary carcinoma.
Wu W, Zhang L, Lin J, Huang H, Shi B, Lin X, Huang Z, Wang C, Qiu J, Wei X. Wu W, et al. Oncotarget. 2016 Dec 20;7(51):84416-84427. doi: 10.18632/oncotarget.12936. Oncotarget. 2016. PMID: 27793057 Free PMC article. - Effects of hypoxia on relationships between cytosolic and mitochondrial NAD(P)H redox and superoxide generation in coronary arterial smooth muscle.
Gao Q, Wolin MS. Gao Q, et al. Am J Physiol Heart Circ Physiol. 2008 Sep;295(3):H978-H989. doi: 10.1152/ajpheart.00316.2008. Epub 2008 Jun 20. Am J Physiol Heart Circ Physiol. 2008. PMID: 18567707 Free PMC article. - Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells.
Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Suchankova G, et al. Biochem Biophys Res Commun. 2009 Jan 23;378(4):836-41. doi: 10.1016/j.bbrc.2008.11.130. Epub 2008 Dec 9. Biochem Biophys Res Commun. 2009. PMID: 19071085 Free PMC article.
References
- Blander G, Guarente L. Annu Rev Biochem. 2004;73:417–435. - PubMed
- Lin SJ, Defossez PA, Guarente L. Science. 2000;289:2126–2128. - PubMed
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Nature. 2002;418:344–348. - PubMed
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Nature. 2000;403:795–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA115468/CA/NCI NIH HHS/United States
- P01 DK044239/DK/NIDDK NIH HHS/United States
- K01 CA 096561/CA/NCI NIH HHS/United States
- P01 DK 044239/DK/NIDDK NIH HHS/United States
- R01 CA115468-05/CA/NCI NIH HHS/United States
- K01 CA096561/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases