Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5 - PubMed (original) (raw)
Comparative Study
Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5
Gareth J O Evans et al. J Neurosci. 2007.
Abstract
The stimulated dephosphorylation of the dephosphin group of endocytic proteins by calcineurin and their subsequent rephosphorylation by cyclin-dependent kinase 5 (cdk5) is required for synaptic vesicle (SV) retrieval in central nerve terminals. However, the specific endocytic pathway(s) controlled by these enzymes is unknown. To address this issue, we combined functional and morphological assays of endocytosis in primary neuronal cultures with pharmacological and molecular ablation of calcineurin and cdk5 activity. During strong stimulation, inhibition of calcineurin or cdk5 blocked uptake of the activity-dependent membrane marker FM1-43, but not the more hydrophilic FM2-10. However, FM2-10 uptake-measured poststimulation was sensitive to cdk5 and calcineurin inhibition, indicating that a slow form of endocytosis persists after termination of stimulation. In parallel EM studies, inhibition of cdk5 during strong stimulation greatly reduced horseradish peroxidase labeling of plasma membrane-derived nerve terminal endosomes, but not SVs. Furthermore, during mild stimulation, FM1-43 uptake was unaffected by cdk5 inhibition and the SV membrane was exclusively retrieved via a single SV route, suggesting that recruitment of the endosomal route of membrane retrieval is activity dependent. Thus, we propose that the calcineurin/cdk5-dependent phosphorylation cycle of the dephosphins specifically controls a slow endocytic pathway that proceeds via endosomal intermediates and is activated by strong physiological stimulation in central nerve terminals.
Figures
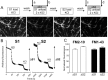
Figure 1.
S2/S1 protocol for investigating SV endocytosis. A, Granule neuron cultures were loaded and unloaded with FM dyes using the protocol displayed. In both S1 and S2, dyes were loaded for 2 min with 50 m
m
KCl and then immediately washed away. Unloading was stimulated by two sequential 30 s stimuli of 50 m
m
KCl. a–d, Representative images of cultures either loaded at S1 (a), unloaded at S1 (b), loaded at S2 (c), or unloaded at S2 (d). Arrows in A indicate the time points at which images were acquired. B, Representative trace showing the average unloading response from 90 nerve terminals in one experiment using FM2–10 during both S1 and S2. Bars indicate addition of KCl. The extent of SV turnover is estimated from the total amount of dye unloading at S1 (ΔS1) and S2 (ΔS2). Error bars indicate SEM. C, The amount of dye unloading at ΔS1 (control) and ΔS2 (test) in the absence of any perturbation of the system is approximately equal for both FM2–10 (open bars ± SEM; n = 233) and FM1–43 (solid bars ± SEM; n = 258). Fluorescence change normalized to ΔS1 for both FM2–10 and FM1–43.
Figure 2.
Antagonism of calcineurin inhibits uptake of FM1–43 but not FM2–10. A, Cultures were loaded and unloaded with either FM2–10 or FM1–43 using the same protocol as in Figure 1_A_. Where indicated, cultures were preincubated with 10 μ
m
CsA for 10 min before and during S2 loading. B, C, Cumulative histograms of the effect of CsA on either FM2–10 (B) or FM1–43 (C) loading in individual nerve terminals (ΔS2/ΔS1, n = 233, FM2–10 control; n = 180, FM2–10 CsA; n = 258, FM1–43 control; n = 168, FM1–43 CsA). D, Cultures were loaded and unloaded with either FM2–10 or FM1–43 using the same protocol as in Figure 1_A_. Where indicated, cultures were preincubated with 10 μ
m
CsA for 10 min before and during S2 unloading. E, F, Cumulative histograms of the effect of CsA on either FM2–10 (E) or FM1–43 (F) unloading in individual nerve terminals (ΔS2/ΔS1, n = 270, FM2–10 CsA; n = 270, FM1–43 CsA). Open symbols, FM2–10; closed symbols, FM1–43; gray symbols, CsA. For all experiments, n = 3.
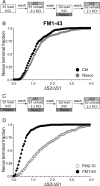
Figure 3.
Delay of FM2–10 washout reveals calcineurin-dependent slow endocytosis. A, FM2–10 was loaded using an identical protocol to Figure 1_A_ except wash of dye was delayed 5 min after termination of KCl stimulation at both S1 and S2. Where indicated, cultures were preincubated with 10 μ
m
CsA for 10 min before and during S2 loading. B, Cumulative histogram of the effect of CsA on dye loading in individual nerve terminals (ΔS2/ΔS1, n = 270 for both Ctrl and CsA). Open symbols, Control (Ctrl); gray symbols, CsA. For all experiments, n = 3.
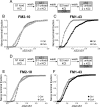
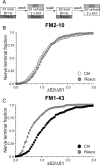
Figure 4.
Antagonism of cdk5 inhibits uptake of FM1–43, but not FM2–10. A, Cultures were loaded and unloaded with FM1–43 using the same protocol as in Figure 1_A_. Where indicated, cultures were preincubated with 50 μ
m
roscovitine (Rosco) for 10 min before and during S2 loading. B, Cumulative histogram of the effect of roscovitine on FM1–43 loading in individual nerve terminals (ΔS2/ΔS1, n = 258, Ctrl; n = 270, Rosco). Closed symbols, Control (Ctrl); gray symbols, roscovitine. C, Cultures were loaded and unloaded with either FM2–10 and FM1–43 using an identical protocol to Figure 1_A_ except that cultures were preincubated with 50 μ
m
roscovitine for 10 min before and during all steps before and including S2 loading. D, Cumulative histogram of the effect of roscovitine on dye loading in individual nerve terminals (ΔS2/ΔS1, n = 209, FM2–10; n = 337, FM1–43). Open symbols represent FM2–10 uptake whereas closed symbols represent FM1–43 uptake both in roscovitine-treated cultures. For all experiments, n = 3, apart from n = 4 for FM1–43 data in D.
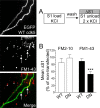
Figure 5.
Overexpression of dominant-negative cdk5 inhibits uptake of FM1–43, but not FM2–10. A, Cultures transfected with either WT or DN cdk5 were loaded and unloaded (S1) with either FM2–10 and FM1–43 using 50 m
m
KCl. Representative images show granule neuron cultures transfected with WT cdk5 and loaded with FM1–43. Merged image shows transfected neuron (green) and FM1–43-loaded nerve terminals (red). Arrows indicate transfected nerve terminals and asterisks indicate untransfected nerve terminals. Note the equal loading of both. B, ΔS1 response in transfected nerve terminals is displayed normalized to the S1 from nontransfected nerve terminals in the same field of view. Open bars, FM2–10; closed bars, FM1–43 (n = 31, WT cdk5 FM2–10; n = 50, DN cdk5 FM2–10; n = 63, WT cdk5 FM1–43; n = 35, DN cdk5 FM1–43; ***p < 0.001, Student's t test). Error bars indicate SEM. For all experiments, n = 3.
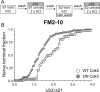
Figure 6.
Overexpression of dominant-negative cdk5 inhibits the delayed uptake of FM2–10. A, FM2–10 was loaded using an identical protocol to Figure 1_A_ except wash of dye was delayed for 5 min after termination of stimulation at S2. B, Cumulative histogram of the effect of either WT or DN cdk5 on dye loading in individual nerve terminals (ΔS2/ΔS1, n = 35, WT cdk5; n = 76, DN cdk5). Open symbols, WT cdk5 transfected nerve terminals; gray symbols, DN cdk5-transfected nerve terminals. In both experiments, n = 3.
Figure 7.
Antagonism of cdk5 inhibits uptake of FM1–43, but not FM2–10 during strong physiological stimulation. A, Cultures were loaded with either FM2–10 or FM1–43 using 800 action potentials delivered at 80 Hz. Dye was washed away immediately after stimulation. Unloading was stimulated with two sequential 30 s stimuli of 50 m
m
KCl. Cultures were preincubated with 50 μ
m
roscovitine (Rosco) for 10 min before and during all steps up to and including S2 loading. B, C, Cumulative histograms of the effect of roscovitine on either FM2–10 (B) or FM1–43 (C) loading in individual nerve terminals are displayed (ΔS2/ΔS1, n = 356 control FM2–10; n = 141, Rosco FM2–10; n = 262, control FM1–43; n = 252, Rosco FM1–43). Open symbols, FM2–10; closed symbols, FM1–43; gray symbols, roscovitine. For all experiments, n = 3, except for n = 4 for FM1–43 Rosco.
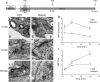
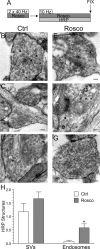
Figure 8.
Inhibition of cdk5 selectively inhibits endosome generation and not single SV endocytosis. A, Cultures were stimulated for 10 s with 50 m
m
KCl, repolarized for 10 min, and then stimulated again with 50 m
m
KCl for 2 min in the presence of HRP. Cultures were repolarized for up to 15 min in the absence of HRP. Cultures were fixed at regular intervals during this time course and were preincubated with 50 μ
m
roscovitine (Rosco) for 10 min before the first KCl pulse and at all steps up to fixation where indicated. B–G, Representative electron micrographs of HRP uptake in control (B–D) or roscovitine-treated (E–G) nerve terminals at 1, 5, and 15 min after stimulation. Black arrows indicate HRP-labeled endosomal structures whereas white arrows indicate HRP-labeled SVs. Scale bars: 200 nm. H, Mean number of HRP-labeled endosomes per nerve terminal. I, Mean number of HRP-labeled SVs per nerve terminal. In H and I, open circles represent control and closed circles represent roscovitine (Ctrl: 1 min, n = 99 nerve terminals; 5 min, n = 213; 15 min, n = 107; Rosco: 1 min, n = 100; 5 min, n = 68; 15 min, n = 43). Error bars indicate SEM.
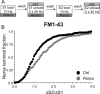
Figure 9.
Cdk5 is not required for endocytosis evoked by mild stimulation. A, FM1–43 was loaded using 200 action potentials delivered at 10 Hz and immediately washed away after termination of stimulation. Dye unloading was evoked by two sequential stimuli of 400 action potentials delivered at 40 Hz. Cultures were preincubated with 50 μ
m
roscovitine (Rosco) for 10 min before and during all steps up to and including S2 loading. B, Cumulative histogram of the effect of roscovitine on dye loading in individual nerve terminals (ΔS2/ΔS1, n = 250, Ctrl; n = 322, Rosco). Closed symbols represent control (Ctrl) and gray symbols represent roscovitine. In all experiments, n = 3 for control and n = 4 for roscovitine.
Figure 10.
Mild stimulation evokes a specific single SV endocytosis pathway independent of cdk5 activity. A, Cultures were stimulated twice for 10 s with two trains of 400 action potentials (40 Hz), repolarized for 10 min, and then stimulated again with a train of 200 action potentials (10 Hz) in the presence of HRP. HRP was kept present for an additional 60 s to ensure labeling of all retrieval pathways and then cultures were fixed. Cultures were preincubated with 50 μ
m
roscovitine (Rosco) for 10 min before the first stimulations and at all steps up to fixation where indicated. B–G, Representative electron micrographs of HRP uptake in control (B–D) or roscovitine-treated (E–G) nerve terminals. Scale bars, 200 nm. Five roscovitine nerve terminals were discounted because they had an atypical number of HRP endosomes compared with the rest (total = 57). H, Mean number of HRP-labeled structures per nerve terminal for either control (open bars) or roscovitine treated (gray bars) cultures ± SEM (Ctrl, n = 29; Rosco, n = 51; *p < 0.05, Student's t test).
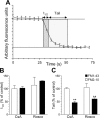
Figure 11.
Inhibition of slow endocytosis results in elimination of a sustained phase of SV exocytosis. A, Two parameters were monitored with respect to dye unloading, the time for nerve terminals to lose 50% of their dye content (t1/2, shown in dark gray) and the time for the remainder to be unloaded (Tail, shown in light gray). In all experiments, dye was loaded with 50 m
m
KCl and washed away immediately after stimulation as in Figure 1_A_. Dye was unloaded with 50 m
m
KCl. Cultures were preincubated with either 10 μ
m
CsA before and during S2 loading or 50 μ
m
Rosco at all steps including S2 loading. B, Effect of CsA and roscovitine (Rosco) on the time taken to unload the initial 50% of dye content (t1/2). C, Effect of CsA and Rosco on the time taken to unload the remainder of dye (Tail). Data are presented as either t1/2 or tail kinetics at S2 normalized to the internal S1 control (n = 6 experiments for FM2–10 CsA; n = 3 for FM2–10 Rosco; n = 4 for FM1–43 CsA; n = 3 for FM1–43 Rosco; all data are ±SEM; Student's t test, **p < 0.01).
Similar articles
- Cdk5 is essential for synaptic vesicle endocytosis.
Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ. Tan TC, et al. Nat Cell Biol. 2003 Aug;5(8):701-10. doi: 10.1038/ncb1020. Nat Cell Biol. 2003. PMID: 12855954 - Activity-dependent bulk endocytosis and clathrin-dependent endocytosis replenish specific synaptic vesicle pools in central nerve terminals.
Cheung G, Jupp OJ, Cousin MA. Cheung G, et al. J Neurosci. 2010 Jun 16;30(24):8151-61. doi: 10.1523/JNEUROSCI.0293-10.2010. J Neurosci. 2010. PMID: 20554865 Free PMC article. - Synaptic vesicle generation from activity-dependent bulk endosomes requires calcium and calcineurin.
Cheung G, Cousin MA. Cheung G, et al. J Neurosci. 2013 Feb 20;33(8):3370-9. doi: 10.1523/JNEUROSCI.4697-12.2013. J Neurosci. 2013. PMID: 23426665 Free PMC article. - Cdk5 and the mystery of synaptic vesicle endocytosis.
Nguyen C, Bibb JA. Nguyen C, et al. J Cell Biol. 2003 Nov 24;163(4):697-9. doi: 10.1083/jcb.200310038. J Cell Biol. 2003. PMID: 14638853 Free PMC article. Review. - The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis.
Cousin MA, Robinson PJ. Cousin MA, et al. Trends Neurosci. 2001 Nov;24(11):659-65. doi: 10.1016/s0166-2236(00)01930-5. Trends Neurosci. 2001. PMID: 11672811 Review.
Cited by
- Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission.
Chung C, Barylko B, Leitz J, Liu X, Kavalali ET. Chung C, et al. J Neurosci. 2010 Jan 27;30(4):1363-76. doi: 10.1523/JNEUROSCI.3427-09.2010. J Neurosci. 2010. PMID: 20107062 Free PMC article. - Loss of myosin Vb promotes apical bulk endocytosis in neonatal enterocytes.
Engevik AC, Kaji I, Postema MM, Faust JJ, Meyer AR, Williams JA, Fitz GN, Tyska MJ, Wilson JM, Goldenring JR. Engevik AC, et al. J Cell Biol. 2019 Nov 4;218(11):3647-3662. doi: 10.1083/jcb.201902063. Epub 2019 Sep 27. J Cell Biol. 2019. PMID: 31562230 Free PMC article. - Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - CHP1 reduction ameliorates spinal muscular atrophy pathology by restoring calcineurin activity and endocytosis.
Janzen E, Mendoza-Ferreira N, Hosseinibarkooie S, Schneider S, Hupperich K, Tschanz T, Grysko V, Riessland M, Hammerschmidt M, Rigo F, Bennett CF, Kye MJ, Torres-Benito L, Wirth B. Janzen E, et al. Brain. 2018 Aug 1;141(8):2343-2361. doi: 10.1093/brain/awy167. Brain. 2018. PMID: 29961886 Free PMC article. - Protein interactions of the vesicular glutamate transporter VGLUT1.
Santos MS, Foss SM, Park CK, Voglmaier SM. Santos MS, et al. PLoS One. 2014 Oct 15;9(10):e109824. doi: 10.1371/journal.pone.0109824. eCollection 2014. PLoS One. 2014. PMID: 25334008 Free PMC article.
References
- Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. - PubMed
- Cousin MA. Synaptic vesicle endocytosis: calcium works overtime in the nerve terminal. Mol Neurobiol. 2000;22:115–128. - PubMed
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources