The C. elegans RSA complex localizes protein phosphatase 2A to centrosomes and regulates mitotic spindle assembly - PubMed (original) (raw)
. 2007 Jan 12;128(1):115-27.
doi: 10.1016/j.cell.2006.10.050.
Martin Srayko, Alexander Dammermann, Sophie Quintin, Natalie Wielsch, Ian MacLeod, Quentin de Robillard, Andrea Zinke, John R Yates 3rd, Thomas Müller-Reichert, Andrei Shevchenko, Karen Oegema, Anthony A Hyman
Affiliations
- PMID: 17218259
- PMCID: PMC2987564
- DOI: 10.1016/j.cell.2006.10.050
The C. elegans RSA complex localizes protein phosphatase 2A to centrosomes and regulates mitotic spindle assembly
Anne-Lore Schlaitz et al. Cell. 2007.
Abstract
Microtubule behavior changes during the cell cycle and during spindle assembly. However, it remains unclear how these changes are regulated and coordinated. We describe a complex that targets the Protein Phosphatase 2A holoenzyme (PP2A) to centrosomes in C. elegans embryos. This complex includes Regulator of Spindle Assembly 1 (RSA-1), a targeting subunit for PP2A, and RSA-2, a protein that binds and recruits RSA-1 to centrosomes. In contrast to the multiple functions of the PP2A catalytic subunit, RSA-1 and RSA-2 are specifically required for microtubule outgrowth from centrosomes and for spindle assembly. The centrosomally localized RSA-PP2A complex mediates these functions in part by regulating two critical mitotic effectors: the microtubule destabilizer KLP-7 and the C. elegans regulator of spindle assembly TPXL-1. By regulating a subset of PP2A functions at the centrosome, the RSA complex could therefore provide a means of coordinating microtubule outgrowth from centrosomes and kinetochore microtubule stability during mitotic spindle assembly.
Figures
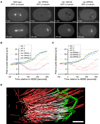
Figure 1. Spindle assembly fails in C. elegans embryos defective in the PP2A regulatory subunit RSA-1 and in embryos depleted of RSA-2
(A) Still images from spinning disk confocal time-lapse recordings of wild-type (first column), rsa-1(RNAi) (second column), rsa-1(dd13) (third column) and rsa-2(RNAi) (fourth column) one-cell stage C. elegans embryos expressing GFP∷β-tubulin. Time points are relative to nuclear envelope breakdown (NEBD=0 seconds). Scale bars are 10µm. See also supplemental movie S1. (B), (C) Measurement of pole-to-pole distances over time during spindle formation from single frames of time-lapse recordings as in (A). Measurements from three wild-type (WT) embryos (green and blue diamonds) and three rsa-1(RNAi) (B) or rsa-2(RNAi) (C) embryos (red and orange circles) are shown. (D) Microtubules contacting the chromatin form in the absence of RSA-1. The 3D model shows a half spindle in anaphase. The partial reconstruction was computed from a 3 × 1 montage. The 3D model shows the boundaries of chromosomes (green) and the position of spindle microtubules (red and white lines). Microtubules that ended on the chromosomes were defined as kinetochore microtubules (white). Scale bar: 1µm
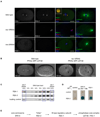
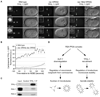
Figure 2. A linear assembly hierarchy targets the RSA-PP2A complex to centrosomes
(A) Centrosomal localization of RSA-1 depends on RSA-2. Wild-type, rsa-1(RNAi) and rsa-2(RNAi) embryos were stained for DNA (blue), RSA-1 (green), RSA-2 (red) and microtubules (green in right panels). Projections of Z-stacks are shown. The inset in the merged image of the top panel highlights RSA-1 and RSA-2 co-localization at centrosomes. Panels on the right illustrate the RNAi phenotypes and the position of the centrosomes. Scale bars are 10µm. inset scale bar is 1µm. (B) The PP2A catalytic subunit LET-92 is recruited to centrosomes by RSA-1. Paired fluorescence and DIC still images from time lapse series of metaphase embryos expressing the GFP tagged PP2A catalytic subunit LET-92. A wild-type embryo is shown in the left panel, a rsa-1(RNAi) embryo is shown in the right panel. Arrows indicate the position of centrosomes in the rsa-1(RNAi) panel as obtained from corresponding DIC recordings. Times indicated are seconds relative to NEBD. Scale bars are 10µm. See supplemental movie S2. (C) The mislocalization of RSA-1 in rsa-2(RNAi) does not appear to be caused by destabilization of the protein. Western blot of whole worm samples of wild-type, rsa-1(RNAi) and rsa-2(RNAi) animals. Both the RSA-1 and the RSA-2 antibodies recognize a protein doublet. In the case of RSA-1, only the upper band (arrow) is specific for the protein, as the lower band is RNAi-resistant. A non-specific reactivity of the RSA-2 antibody served as loading control. (D) RSA-2 interacts with RSA-1 and SPD-5 in a yeast-two-hybrid assay. A vector encoding RSA-2 fused to the GAL4 DNA binding domain (BD) was co-transformed with GAL4 activating domain (AD) fusions of either full-length RSA-1 or a SPD-5 fragment that corresponded to amino acids 280–1198. Complex formation was detected by activation of the lacZ reporter gene in a β-galactosidase assay. See supplemental table 1 for a quantification of β-galactosidase units. Complex formation was further confirmed by use of two additional reporters: growth on plates lacking histidine or uracil, respectively (data not shown). (E) Scheme of the linear assembly hierarchy that targets the PP2A complex to centrosomes.
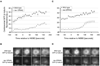
Figure 3. Depletion of RSA-1 or RSA-2 causes a reduction of centrosomal microtubules
(A) and (C) Quantification of centrosomal GFP∷β-tubulin fluorescence from single frames of time-lapse recordings as in figure 1A. Fluorescence intensity values are shown as (mean ± standard error of the mean) for wild-type (diamonds) and rsa-1(RNAi) (A) or rsa-2(RNAi) (C) embryos (circles). (B) and (D) Representative images of centrosomes of rsa-1(RNAi) (B) and rsa-2(RNAi) (D) embryos acquired as in figure 1A. Scale bars are 1µm. Time points indicated are relative to NEBD.
Figure 4. Protein phosphatase activity is required for centrosomal microtubule stability
(A) Depletion of the PP2A catalytic subunit LET-92 leads to pleiotropic defects in the early embryo and to reduced centrosomal microtubules. Embryos expressing GFP∷β-tubulin were depleted of the PP2A catalytic subunit LET-92 and imaged by spinning disk confocal microscopy. Still images representing cell cycle progression (panels I to IV) in let-92(RNAi) embryos are shown. Panel I: Meiotic spindle remnants (arrowhead) and the unseparated centrosomes (arrow) are at the embryonic cortex. Panel II: Meiotic spindle remnants and centrosomes migrate towards the centre of the embryo where they meet. Panel III: The meiotic microtubule array and the centrosomes fuse and form a bipolar structure. Panel IV: The bipolar structure elongates. The right panel shows a still image of a wild-type embryo at anaphase acquired under the same conditions for comparison of microtubule amounts with panel IV of the let-92(RNAi) series. See supplemental movie S4. (B) Microtubules are destabilized in embryos treated with Calyculin A, an inhibitor of Protein Phosphatase 2A and Protein Phosphatase 1. YFP∷α-tubulin embryos were mounted with 10 µm Calyculin A diluted from a 1 mM DMSO stock (DMSO/Calyculin A embryo, lower panels) or with 1% v/v DMSO alone (DMSO control embryos, upper panels). An image of the cellular microtubules before drug treatment was acquired (left panels). After laser-mediated eggshell permeabilization and entry of Calyculin A into the embryonic cytoplasm (circle marks the site of eggshell perforation), microtubule behavior was followed using one-second interval time-lapse acquisitions. Time points indicated are seconds after eggshell perforation and drug entry (right panels). Out of 15 embryos that were exposed to Calyculin A, 10 showed a rapid microtubule depolymerization like the embryo shown in this figure, 5 embryos displayed a slower loss of microtubules, which might be caused by slightly different cell cycle stages at the time of drug entry or by less access of Calyculin A to the cytoplasm of these embryos. (A) and (B) Scale bars are 10µm. See supplemental movie S5.
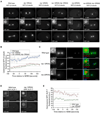
Figure 5. Microtubule reduction in rsa-1(RNAi) and rsa-2(RNAi) depends on KLP-7
(A) Microtubule reduction in rsa-1(RNAi) and rsa-2(RNAi) can be overcome by co-depletion of the microtubule depolymerizing kinesin KLP-7. Still images of time-lapse series of GFP∷β-tubulin expressing embryos of wild type, klp-7(RNAi) and rsa-1(RNAi) single depletions, rsa-1(RNAi); klp-7(RNAi) double depletion as well as rsa-2(RNAi) single depletion and rsa-2(RNAi); klp-7(RNAi) double depletion. Aspects of both single depletion phenotypes are observable in the double RNAi experiments: spindle collapse marking rsa-1(RNAi) and rsa-2(RNAi), respectively, and enlarged polar bodies (arrowheads) indicating efficient removal of KLP-7. Double RNAi experiments were controlled by dilution of single dsRNAs with an unspecific dsRNA. See supplemental movies 6 and 7. (B) Quantification of centrosomal GFP∷β-tubulin fluorescence from single frames of time-lapse recordings as in (A). Measurements are displayed as (mean values ± SEM) for wild type embryos (green diamonds), klp-7(RNAi) embryos (blue squares) and rsa-1(RNAi); klp-7(RNAi) double RNAi embryos (red circles). Microtubule amounts for RSA-1 single depletion could not be quantified in this experiment as exposure times had to be decreased to avoid overexposure of the brighter klp-7(RNAi) centrosomes, so that rsa-1(RNAi) centrosomes were too dim for quantifications. See figure 3A for comparison of rsa-1(RNAi) and wild-type microtubule amounts. (C) γ-tubulin localizes normally in rsa-1(RNAi) and rsa-2(RNAi) embryos. Wild-type, rsa-1(RNAi) and rsa-2(RNAi) embryos were fixed and stained for DNA (blue), microtubules (green) and γ-tubulin (red). Z-stack projections are shown. See also supplemental figure 4B for a quantification of γ-tubulin levels in rsa-1(RNAi). (D) Centrosomal levels of KLP-7 are increased in rsa-1(RNAi). Still images of time-lapse series of wild-type and rsa-1(RNAi) embryos expressing GFP∷KLP-7. See also supplemental movie S9. (E) Quantification of centrosomal GFP∷KLP-7 fluorescence in wild-type (WT) embryos (green diamonds) and rsa-1(RNAi) embryos (red circles) from single frames of time-lapse recordings as in (D). Values are mean ± SEM. (A) and (D) Times indicated are seconds relative to NEBD. (A), (C) and (D) Scale bars are 10µm; inset scale bars are 1µm.
Figure 6. RSA-1 is required for the centrosomal localization of TPXL-1
(A) Still images taken from time lapse series of wild-type, rsa-1(RNAi), and rsa-1(RNAi); klp-7(RNAi) double depletion embryos expressing TPXL-1∷GFP. Paired fluorescence and DIC images are shown. Arrows indicate the positions of the centrosomes in rsa-1(RNAi) and rsa-1(RNAi); klp-7(RNAi). Successful depletion of KLP-7 is indicated by an enlarged polar body (arrowhead). Time points are relative to NEBD. Scale bars are 10µm. See supplemental movie S10. (B) Quantification of centrosomal TPXL-1∷GFP amounts from single frames of time lapse recordings as shown in (A). Mean values ± SEM are shown for wild type (green diamonds) and rsa-1(RNAi); klp-7(RNAi) centrosomes (red circles). (C) TPXL-1 associates with the RSA complex. TPXL-1 was immunoprecipitated from C. elegans embryo extracts. Co-purifying proteins were identified by Western blotting and antibody detection. In a different IP experiment, the association of TPXL-1 was confirmed by mass spectrometric analysis (supplemental table 2) (D) The RSA-PP2A complex regulates two separate pathways in spindle formation. Downregulation of KLP-7 by RSA-PP2A allows the correct number of microtubules to grow out from centrosomes (left branch). Recruitment of TPXL-1 to centrosomes by the RSA-PP2A complex is required for stabilization of kinetochore microtubules (grey) and thereby spindle stability (right branch).
Similar articles
- Kinetochore Recruitment of the Spindle and Kinetochore-Associated (Ska) Complex Is Regulated by Centrosomal PP2A in Caenorhabditis elegans.
Lange KI, Suleman A, Srayko M. Lange KI, et al. Genetics. 2019 Jun;212(2):509-522. doi: 10.1534/genetics.119.302105. Epub 2019 Apr 24. Genetics. 2019. PMID: 31018924 Free PMC article. - C. elegans chromosomes connect to centrosomes by anchoring into the spindle network.
Redemann S, Baumgart J, Lindow N, Shelley M, Nazockdast E, Kratz A, Prohaska S, Brugués J, Fürthauer S, Müller-Reichert T. Redemann S, et al. Nat Commun. 2017 May 11;8:15288. doi: 10.1038/ncomms15288. Nat Commun. 2017. PMID: 28492281 Free PMC article. - LET-711, the Caenorhabditis elegans NOT1 ortholog, is required for spindle positioning and regulation of microtubule length in embryos.
DeBella LR, Hayashi A, Rose LS. DeBella LR, et al. Mol Biol Cell. 2006 Nov;17(11):4911-24. doi: 10.1091/mbc.e06-02-0107. Epub 2006 Sep 13. Mol Biol Cell. 2006. PMID: 16971515 Free PMC article. - Centrosomes and the art of mitotic spindle maintenance.
Hinchcliffe EH. Hinchcliffe EH. Int Rev Cell Mol Biol. 2014;313:179-217. doi: 10.1016/B978-0-12-800177-6.00006-2. Int Rev Cell Mol Biol. 2014. PMID: 25376493 Review. - Physical Limits on the Precision of Mitotic Spindle Positioning by Microtubule Pushing forces: Mechanics of mitotic spindle positioning.
Howard J, Garzon-Coral C. Howard J, et al. Bioessays. 2017 Nov;39(11):10.1002/bies.201700122. doi: 10.1002/bies.201700122. Epub 2017 Sep 28. Bioessays. 2017. PMID: 28960439 Free PMC article. Review.
Cited by
- A protein phosphatase 2A complex spatially controls plant cell division.
Spinner L, Gadeyne A, Belcram K, Goussot M, Moison M, Duroc Y, Eeckhout D, De Winne N, Schaefer E, Van De Slijke E, Persiau G, Witters E, Gevaert K, De Jaeger G, Bouchez D, Van Damme D, Pastuglia M. Spinner L, et al. Nat Commun. 2013;4:1863. doi: 10.1038/ncomms2831. Nat Commun. 2013. PMID: 23673648 - Nematode gene annotation by machine-learning-assisted proteotranscriptomics enables proteome-wide evolutionary analysis.
Ceron-Noriega A, Almeida MV, Levin M, Butter F. Ceron-Noriega A, et al. Genome Res. 2023 Jan;33(1):112-128. doi: 10.1101/gr.277070.122. Epub 2023 Jan 18. Genome Res. 2023. PMID: 36653121 Free PMC article. - Whole genome sequencing identifies a deletion in protein phosphatase 2A that affects its stability and localization in Chlamydomonas reinhardtii.
Lin H, Miller ML, Granas DM, Dutcher SK. Lin H, et al. PLoS Genet. 2013;9(9):e1003841. doi: 10.1371/journal.pgen.1003841. Epub 2013 Sep 26. PLoS Genet. 2013. PMID: 24086163 Free PMC article. - sSgo1, a major splice variant of Sgo1, functions in centriole cohesion where it is regulated by Plk1.
Wang X, Yang Y, Duan Q, Jiang N, Huang Y, Darzynkiewicz Z, Dai W. Wang X, et al. Dev Cell. 2008 Mar;14(3):331-41. doi: 10.1016/j.devcel.2007.12.007. Dev Cell. 2008. PMID: 18331714 Free PMC article. - The benefits of local depletion: The centrosome as a scaffold for ubiquitin-proteasome-mediated degradation.
Vora SM, Phillips BT. Vora SM, et al. Cell Cycle. 2016 Aug 17;15(16):2124-2134. doi: 10.1080/15384101.2016.1196306. Epub 2016 Jun 13. Cell Cycle. 2016. PMID: 27294844 Free PMC article. Review.
References
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. - PubMed
- Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases