Neuronal oscillations and multisensory interaction in primary auditory cortex - PubMed (original) (raw)
Neuronal oscillations and multisensory interaction in primary auditory cortex
Peter Lakatos et al. Neuron. 2007.
Abstract
Recent anatomical, physiological, and neuroimaging findings indicate multisensory convergence at early, putatively unisensory stages of cortical processing. The objective of this study was to confirm somatosensory-auditory interaction in A1 and to define both its physiological mechanisms and its consequences for auditory information processing. Laminar current source density and multiunit activity sampled during multielectrode penetrations of primary auditory area A1 in awake macaques revealed clear somatosensory-auditory interactions, with a novel mechanism: somatosensory inputs appear to reset the phase of ongoing neuronal oscillations, so that accompanying auditory inputs arrive during an ideal, high-excitability phase, and produce amplified neuronal responses. In contrast, responses to auditory inputs arriving during the opposing low-excitability phase tend to be suppressed. Our findings underscore the instrumental role of neuronal oscillations in cortical operations. The timing and laminar profile of the multisensory interactions in A1 indicate that nonspecific thalamic systems may play a key role in the effect.
Figures
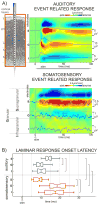
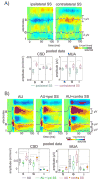
Figure 1. Laminar profiles of auditory and somatosensory event related responses in area AI of the auditory cortex
A) Field potentials (used to calculate the CSD) and MUA were recorded concomitantly with a linear-array multi-contact electrode positioned to sample from all cortical layers. Laminar boundaries were determined based on functional criteria (see Experimental Procedures). Color-maps show the laminar profiles of a representative characteristic frequency tone and a somatosensory stimulus related averaged CSD (98 and 95 sweeps respectively), recorded in the same location. Current sinks (net inward transmembrane current) are red and current sources (net outward transmembrane current) are blue. Based on their largest amplitude in the auditory CSD one electrode was selected in each layer (S, G, and I) for quantitative analysis. Overlaid traces show MUA in the selected channels. B) Box-plots show pooled onset latencies of the characteristic frequency tone (blue) and somatosensory stimulus (red) related CSD in the selected channels for all experiments. The boxes have lines at the lower quartile, median, and upper quartile values while the notches in boxes graphically show the 95% confidence interval about the median of each distribution. Brackets indicate the significant post hoc comparisons calculated using Games-Howell tests (p<0.01).
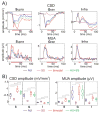
Figure 2. Auditory, somatosensory and bimodal event related responses
A) CSD (upper) and MUA (lower) responses to auditory, somatosensory and bimodal stimuli on the selected supragranular (S), granular (G), and infragranular (I) channels (from the same site as Fig. 1). Green dotted line shows the arithmetic sum of the unimodal responses. Red lines on the time-axis denote time intervals where the averaged bimodal responses were significantly (independent-samples t-tests, p<0.01) greater than the sum of the averaged unimodal responses in the pooled data (n=38). B) Box-plots show pooled (n=38) CSD and MUA amplitudes on the selected channels (S, G, and I) averaged for the 15–60 ms time interval for the same conditions as panel A. Brackets indicate the significant post hoc comparisons calculated using Games-Howell tests (p<0.01).
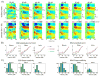
Figure 3. Super-additivity and inverse effectiveness
A) Color-maps show the laminar profiles of auditory (upper) and bimodal (lower) CSD responses at different auditory stimulus intensities. Overlaid traces show MUA in the selected supragranular (S), granular (G), and infragranular (I) channels. B) Line-plots shows single trial CSD and MUA amplitudes on the selected channels (S, G, and I) averaged for the 15–60 ms time interval. Error-bars represent standard error, stars denote where the single trial bimodal response amplitudes were significantly significant larger then the arithmetic sum of the unimodal responses (one-sample t-tests, p<0.01). C) Bar graphs show the percentage of experiments (out of a total of 20) at each auditory intensity, where single trial bimodal response amplitudes (CSD and MUA) were significantly larger then the arithmetic sum of the unimodal responses in each layer.
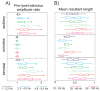
Figure 4. Effect of somatosensory-auditory SOA on the supragranular bimodal response
A) Color-map shows the event related CSD of the supragranular channel (S, see Fig. 1) in area AI for different somatosensory-auditory SOAs. Increasing SOAs are mapped to the y-axis from top to bottom, with 0 on top corresponding to simultaneous auditory-somatosensory stimulation. AU in the bottom represents the auditory alone condition. Red dotted lines denote the 20–60 ms time interval for which we averaged the CSD and MUA in single trials for quantitative analysis. B) Traces show mean CSD and MUA amplitude values (x-axis) for the 20–60 ms auditory post-stimulus time interval (error-bars show standard errors) with different somatosensory-auditory SOAs (y-axis). Blue dotted line denotes the mean amplitude of the auditory alone response. At a given SOA, independent-samples t-tests were used for all six experiments (bimodal response amplitude in each experiment was compared to the response amplitude of the auditory alone condition). The number of stars at a given SOA indicates how many experiments have significant differences (independent-samples t-tests, p<0.01) in bimodal activation.
Figure 5. Ipsi- and contralateral somatosensory event related responses in area AI and their effect on auditory stimulus processing
A) Color-maps show ipsi- and contralateral somatosensory event related CSD profiles. Overlaid traces show MUA in the selected channels for each cortical layer. Box-plots show pooled averaged CSD and MUA response amplitudes to ipsi- and contralateral somatosensory stimuli on the selected channels for the 15–60 ms time interval. Brackets indicate significant differences between ipsilateral and contralateral conditions calculated using independent-samples t-tests (p<0.01). B) Color-maps with overlaid traces show CSD and MUA of unimodal auditory, and bimodal auditory + ipsilateral and auditory + contralateral somatosensory responses. Box-plots show pooled averaged CSD and MUA response amplitudes to unimodal auditory, auditory + ipsilateral and auditory + contralateral somatosensory stimuli on the selected channels for the 15–60 ms time interval. Brackets indicate the significant post hoc comparisons calculated using Games-Howell tests (p<0.01). There was no significant difference between the response amplitudes to auditory + contralateral and auditory + bilateral somatosensory stimuli (for auditory + bilateral somatosensory response amplitudes in the same paradigm see Fig. 2B.)
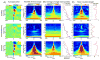
Figure 6. Oscillatory properties of auditory, somatosensory and bimodal responses
A) Color-maps to the left show the laminar profiles of auditory, somatosensory and bimodal event related averaged CSD responses for the −500 to 500 ms timeframe. Time-frequency plots to the right show oscillatory amplitudes of the supragranular (S) averaged responses for the same timeframe (x-axis) with frequency on the logarithmic y-axis. B) Time-frequency plots show the average oscillatory amplitude of the wavelet transformed single trials. The traces to the right show the pre- (blue, −500 –−250 ms) and post-stimulus (red, 0 – 250 ms) amplitudes (x-axis) at different frequencies (y axis). Grey dotted lines indicate the frequency intervals used for quantitative analysis (see Fig. 7). Frequency bands were chosen based on results from previous studies. C) Time-frequency plots show the mean resultant length (R) of the single trial phases at different times/frequencies. This value will be 1 if at a given time point the oscillatory phase is the same in each trial, and will be 0 if the oscillatory phase is random (see Experimental Procedures). Traces to the right show the mean resultant length at 15 ms post-stimulus. Blue dotted line depicts the threshold for significant deviation from a uniform (random) phase distribution (Rayleigh’s uniformity tests, p = 0.01).
Figure 7. Event related single trial oscillatory amplitudes and phase concentration
A) Pooled n=38) post-/pre-stimulus single trial oscillatory amplitude ratio (0 – 250 ms/−500 – −250 ms) for different frequency intervals (different colors) of the auditory, somatosensory and bimodal supragranular responses. Stars denote where the amplitude ratio is significantly different than 1 (one-sample t-tests, p<0.01). B) Pooled mean resultant length values at 15 ms post-stimulus. Note that in the case of somatosensory events, significant phase concentration only occurs in the low-delta (1–2.2 Hz), theta (4.8–9.3 Hz) and gamma (25–49 Hz) bands.
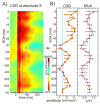
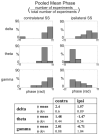
Figure 8. Contra- and ipsilateral somatosensory event related phase at the dominant frequencies
A) Pooled mean delta, theta and gamma oscillatory phase associated with contra- and ipsilateral somatosensory stimulation on the selected supragranular electrode. Mean phase values are derived from single trial wavelet phases at 15 ms post-stimulus (average auditory onset latency in the supragranular layers) in each experiment. Bar graphs show the percentage of experiments (out of a total of 20) where the mean phase fell into a given phase bin (6 bins from −π to π). Table shows the pooled mean phase values of the dominant oscillations and angular deviance of the means at 15 ms post-stimulus.
Comment in
- Paving the way forward: integrating the senses through phase-resetting of cortical oscillations.
Ghazanfar AA, Chandrasekaran CF. Ghazanfar AA, et al. Neuron. 2007 Jan 18;53(2):162-4. doi: 10.1016/j.neuron.2007.01.003. Neuron. 2007. PMID: 17224399
Similar articles
- Paving the way forward: integrating the senses through phase-resetting of cortical oscillations.
Ghazanfar AA, Chandrasekaran CF. Ghazanfar AA, et al. Neuron. 2007 Jan 18;53(2):162-4. doi: 10.1016/j.neuron.2007.01.003. Neuron. 2007. PMID: 17224399 - Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys.
Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE. Smiley JF, et al. J Comp Neurol. 2007 Jun 20;502(6):894-923. doi: 10.1002/cne.21325. J Comp Neurol. 2007. PMID: 17447261 - Somatosensory input to auditory association cortex in the macaque monkey.
Schroeder CE, Lindsley RW, Specht C, Marcovici A, Smiley JF, Javitt DC. Schroeder CE, et al. J Neurophysiol. 2001 Mar;85(3):1322-7. doi: 10.1152/jn.2001.85.3.1322. J Neurophysiol. 2001. PMID: 11248001 - Neuronal mechanisms, response dynamics and perceptual functions of multisensory interactions in auditory cortex.
Musacchia G, Schroeder CE. Musacchia G, et al. Hear Res. 2009 Dec;258(1-2):72-9. doi: 10.1016/j.heares.2009.06.018. Epub 2009 Jul 10. Hear Res. 2009. PMID: 19595755 Free PMC article. Review. - Anatomical mechanisms and functional implications of multisensory convergence in early cortical processing.
Schroeder CE, Smiley J, Fu KG, McGinnis T, O'Connell MN, Hackett TA. Schroeder CE, et al. Int J Psychophysiol. 2003 Oct;50(1-2):5-17. doi: 10.1016/s0167-8760(03)00120-x. Int J Psychophysiol. 2003. PMID: 14511832 Review.
Cited by
- Human hippocampal connectivity is stronger in olfaction than other sensory systems.
Zhou G, Olofsson JK, Koubeissi MZ, Menelaou G, Rosenow J, Schuele SU, Xu P, Voss JL, Lane G, Zelano C. Zhou G, et al. Prog Neurobiol. 2021 Jun;201:102027. doi: 10.1016/j.pneurobio.2021.102027. Epub 2021 Feb 25. Prog Neurobiol. 2021. PMID: 33640412 Free PMC article. - Rhythmic modulation of visual contrast discrimination triggered by action.
Benedetto A, Spinelli D, Morrone MC. Benedetto A, et al. Proc Biol Sci. 2016 May 25;283(1831):20160692. doi: 10.1098/rspb.2016.0692. Proc Biol Sci. 2016. PMID: 27226468 Free PMC article. - Coupled oscillations enable rapid temporal recalibration to audiovisual asynchrony.
Lennert T, Samiee S, Baillet S. Lennert T, et al. Commun Biol. 2021 May 11;4(1):559. doi: 10.1038/s42003-021-02087-0. Commun Biol. 2021. PMID: 33976360 Free PMC article. - Multisensory integration in neurons of the medial pulvinar of macaque monkey.
Vittek AL, Juan C, Nowak LG, Girard P, Cappe C. Vittek AL, et al. Cereb Cortex. 2023 Apr 4;33(8):4202-4215. doi: 10.1093/cercor/bhac337. Cereb Cortex. 2023. PMID: 36068947 Free PMC article. - Audio-visual onset differences are used to determine syllable identity for ambiguous audio-visual stimulus pairs.
Ten Oever S, Sack AT, Wheat KL, Bien N, van Atteveldt N. Ten Oever S, et al. Front Psychol. 2013 Jun 26;4:331. doi: 10.3389/fpsyg.2013.00331. eCollection 2013. Front Psychol. 2013. PMID: 23805110 Free PMC article.
References
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
- Atteveldt N, Formisano E, Goebel R, Blomert L. Integration of letters and speech sounds in the human brain. Neuron. 2004;43:271–282. - PubMed
- Blum PS, Abraham LD, Gilman S. Vestibular, auditory, and somatic input to the posterior thalamus of the cat. Exp Brain Res. 1979;34:1–9. - PubMed
- Callan DE, Callan AM, Kroos C, Vatikiotis-Bateson E. Multimodal contribution to speech perception revealed by independent component analysis: a single-sweep EEG case study. Brain Res Cogn Brain Res. 2001;10:349–353. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources