Alterations of cellular bioenergetics in pulmonary artery endothelial cells - PubMed (original) (raw)
. 2007 Jan 23;104(4):1342-7.
doi: 10.1073/pnas.0605080104. Epub 2007 Jan 16.
Thomas Koeck, Abigail R Lara, Donald Neumann, Frank P DiFilippo, Michelle Koo, Allison J Janocha, Fares A Masri, Alejandro C Arroliga, Constance Jennings, Raed A Dweik, Rubin M Tuder, Dennis J Stuehr, Serpil C Erzurum
Affiliations
- PMID: 17227868
- PMCID: PMC1783136
- DOI: 10.1073/pnas.0605080104
Alterations of cellular bioenergetics in pulmonary artery endothelial cells
Weiling Xu et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Idiopathic pulmonary arterial hypertension (IPAH) is pathogenetically related to low levels of the vasodilator nitric oxide (NO). Because NO regulates cellular respiration and mitochondrial biogenesis, we hypothesized that abnormalities of bioenergetics may be present in IPAH. Evaluation of pulmonary artery endothelial cells from IPAH and control lungs in vitro revealed that oxygen consumption of IPAH cells was decreased, especially in state 3 respiration with substrates glutamate-malate or succinate, and this decrease paralleled reduction in Complex IV activity and IPAH cellular NO synthesis. IPAH pulmonary artery endothelial cells had decreased mitochondrial dehydrogenase activity and lowered mitochondrial numbers per cell and mitochondrial DNA content, all of which increased after exposure to NO donors. Although IPAH/pulmonary artery endothelial cells' ATP content was similar to control under normoxia, cellular ATP did not change significantly in IPAH cells under hypoxia, whereas ATP decreased 35% in control cells, identifying a greater dependence on cellular respiration for energy in control cells. Evidence that glucose metabolism was subserving the primary role for energy requirements of IPAH cells was provided by the approximately 3-fold greater glycolytic rate of IPAH cells. Positron emission tomography scan with [18F]fluoro-deoxy-D-glucose performed on IPAH patients and healthy controls revealed significantly higher uptake in IPAH lungs as compared with controls, confirming that the glycolytic rate was increased in vivo. Thus, there are substantial changes in bioenergetics of IPAH endothelial cells, which may have consequences for pulmonary hypertensive responses and potentially in development of novel imaging modalities for diagnosis and evaluation of treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
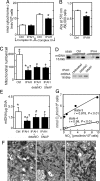
Fig. 1.
Mitochondrial function, amount, and morphology. (A) Activities of mitochondrial Complexes III and IV of IPAH PAEC and healthy controls (*, P < 0.05, n = 9 replicate experiments). (B) Mitochondrial dehydrogenase activity of IPAH and control cells. MTT reduction (Abs at 570 nm per 60,000 cells) by IPAH cells was lower than control (*, P < 0.05, n = 9 replicate experiments). (C) Mitochondrial number per cell by electron microscopy in untreated IPAH and healthy control PAEC and IPAH PAEC exposed to NO donors, DETA NONOate (detaNO), or SNAP (*, #, †, P < 0.01). (D) Southern blot analysis of mtDNA content in IPAH PAEC. (Upper) Each lane contains increasing total DNA from PAEC loaded at 1, 3, or 10 μg or from A549 cells (10 μg). (Lower) Total DNA (5 μg per lane) from IPAH PAEC with NO donors. (E) mtDNA relative units per microgram of DNA in untreated IPAH and healthy control and in IPAH exposed to NO donors (*, †, P < 0.05; #, P = 0.06). (F) Ultrastructure detail of mitochondria in untreated IPAH PAEC (Center), healthy control (Left), and IPAH exposed to detaNO (Right). (Scale bar: 1 μm.) (G) Endogenous NO production and cell respiration. Correlation of nitrite (NO2−) concentrations in the culture supernatants from ionomycin-treated cells (picomoles per minute per 106 cells) and oxygen consumption (nanomoles of O2 per minute per 106 cells) for state 3 (n = 4) and state 4 (n = 5) respirations from concurrent cultured IPAH and healthy control cells. Symbols in figures identify the comparison pairs for multiple comparisons, and the P values are noted in the text.
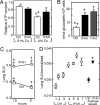
Fig. 2.
ATP content and glycolytic rate in vitro and glucose metabolic activities in vivo. (A) Under normoxia (21% O2), ATP content in IPAH PAEC (n = 4) was similar to controls (n = 3). Under hypoxia (2% O2), ATP content in IPAH PAEC (n = 4) was significantly higher than controls (n = 3) but similar to the value under normoxia (*, †, P < 0.01, n = 14 replicate experiments; ATP values shown are relative to values of control cells under normoxia). (B) Glycolytic rate from IPAH PAEC (n = 5) or from PAH PAEC (n = 4) was higher than healthy controls (n = 3, *, †, P < 0.01). (C) SUV of FDG-PET scan in lungs of IPAH patients (n = 4) and healthy controls (n = 3) at 1.5 and 3 h after injection (*, †, P < 0.01). (D) Glucose metabolic activities in lungs of IPAH patients were higher than in healthy controls (*, P < 0.01) in four IPAH patients (subjects 4–7) as compared with three healthy controls (subjects 1–3). The 3-h SUV was normalized to LTF. Symbols in figures identify the comparison pairs for multiple comparisons, and the P values are noted in the text.
References
- Jeffery TK, Morrell NW. Prog Cardiovasc Dis. 2002;45:173–202. - PubMed
- Archer S, Rich S. Circulation. 2000;102:2781–2791. - PubMed
- Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Am J Respir Crit Care Med. 1998;158:917–923. - PubMed
- Azeka E, Costa Auler JO, Jr, Kajita L, Alliman AC, Franchini Ramires JA, Ebaid M. Pediatr Cardiol. 2002;23:20–26. - PubMed
- Cooke JP, Dzau VJ. Circulation. 1997;96:379–382. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources