Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron - PubMed (original) (raw)
. 2007 Feb;134(4):801-11.
doi: 10.1242/dev.02773. Epub 2007 Jan 17.
Affiliations
- PMID: 17229764
- PMCID: PMC2613851
- DOI: 10.1242/dev.02773
Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron
Hui-Teng Cheng et al. Development. 2007 Feb.
Abstract
The Notch pathway regulates cell fate determination in numerous developmental processes. Here we report that Notch2 acts non-redundantly to control the processes of nephron segmentation through an Rbp-J-dependent process. Notch1 and Notch2 are detected in the early renal vesicle. Genetic analysis reveals that only Notch2 is required for the differentiation of proximal nephron structures (podocytes and proximal convoluted tubules) despite the presence of activated Notch1 in the nuclei of putative proximal progenitors. The inability of endogenous Notch1 to compensate for Notch2 deficiency may reflect sub-threshold Notch1 levels in the nucleus. In line with this view, forced expression of a gamma-secretase-independent form of Notch1 intracellular domain drives the specification of proximal fates where all endogenous, ligand-dependent Notch signaling is blocked by a gamma-secretase inhibitor. These results establish distinct (non-redundant), instructive roles for Notch receptors in nephron segmentation.
Figures
Fig. 1
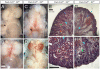
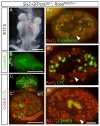
Loss of Notch2 causes hypoplastic kidneys that do not develop glomeruli, proximal tubules and S-shaped bodies. (A to D) The urinary system from postnatal day 1 and day 2 wild type (A, B) or mutant (C, D) animals. Notice the size difference of the urinary bladder (arrow in A and C). The mutant animals show spotty hemorrhage on the kidneys before they die on postnatal day 2 (D). (E to H) Histology of day 2 kidneys from wild type (E, F) and mutant (G, H) stained with H&E. Blue arrows flank the nephrogenic zone. Red arrow: glomerulus. Green arrow: proximal tubule. Yellow arrow: S-shaped body. Turquoise arrowhead: collecting duct. Green arrowhead: presumptive renal tubule in mutant. P: proximal tubule. D: distal tubule. The wild type genotype is Pax3-cretg/+; N2f/+; the mutant genotype is Pax3-cretg/+; fN2/fN2. Bar, 1mm (A to D), 0.1mm (E to H). Red arrows mark glomeruli, green-proximal tubule (marked also with P), turquoise- duct, yellow- S-shaped bodies, dark blue- nephrogenic zone. D indicates distal tubules.
Fig. 2
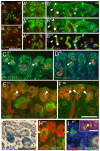
Notch2-deficient kidneys (N2) develop distal tubules without formation of podocytes and proximal tubules. (A, B) Wild type (A) kidney contains high Wilms tumor-1-expressing cells in glomerular podocytes and S-shaped bodies. The only cells that express low level of Wilms tumor-1 in mutant (B) are mesenchymal cells surrounding cytokeratin8-expressing ureteric buds (red). (C, D) No LTL-stained proximal tubules are found in mutant (C), compared to wild type (D). (E, F) Mutant kidney (E) develops numerous E-cadherin-positive, cytokeratin8-negative distal tubules, some of which are connected to cytokeratin8-positive ducts (dash line). The wild type proximal tubules, judged by morphology, also express E-cadherin (arrowhead in F). Wt: wild type. N2: Notch2 mutant. Bar, 0.1mm (A, B, E’) and (C to F).
Fig. 3
(A to E) Notch2-deficient mesenchyme undergoes normal epithelialization but the newly formed nephron fails to resolve into S-shaped body. (A, B) S-shaped bodies are seen in wild type (arrowhead in A). In Notch2 mutant, one nephron, which expresses N-CAM, can be identified at each ureteric bud tip (arrow in B). They synthesize laminin α1 (arrowhead in D), so do the S-shaped bodies (arrowhead in C). (E) Three progressive stages during nephrogenesis in the mutant are marked as 1, 2 and 3. Bar, 0.1mm. (F to J) Each of three segments in the S-shaped body expresses molecular markers (see text). Distal tubule precursors are Pax2high and E-cadherin positive (F, H). Jagged1 is localized in the middle segment where proximal tubule precursors reside (G). WT-1 marks the podocyte precursors (I). N1-ICD is detectable in both the proximal and podocyte precursors (J). Bar, 0.05mm.
Fig. 4
The segmentation process in Notch2-deficient nephron (N2) is impaired. (A, B) Cadherin-6-expressing cells are adjacent to E-cadherin-expressing cells in wild type (A) but no Cadherin-6-expressing cells are detectable in mutant (B). (A’ through B’”) Serial sections are stained with the segmentation markers Pax2, WT-1 and E-cadherin. Arrows and arrowheads indicate similar structure across all sections. (C, D) Lim1 expression pattern in the renal vesicle is similar in both wild type and Notch2 mutant kidneys (white arrow). The cells at the very proximal end of the mutant nephron express high Lim1 (D). This distribution is different from a wild type S-shaped body (C). (E) Few Jagged1-expressing cells are seen in the renal vesicle (arrowhead), and the population expends with the formation of the S-shaped body (circle). A small cluster of Jagged1-expressing cells is seen in each of the mutant nephrons (F). Dll-1LacZ and N1-ICD are detectable in the renal vesicle (G, H). In some cases, Jagged1 is not co-localized with N1-ICD (I). N1-ICD is still detectable in Notch2-deficient nephrons (inset in I). Bar, 0.1mm (A to F), 0.05mm (G-I).
Fig. 5
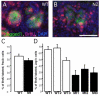
Wild type and Notch2 mutant, Pax2-expressing early renal epithelia are proliferating to a similar degree while Jagged1-expressing, Notch2-deficient clusters have few cycling cells. (A, B) Representative images of BrdU-incorporation (red) within the Jagged1-expressing clusters (green). Bar, 0.05mm. (C, D) Histogram of results from several embryos indicates the percentage of BrdU-labeled cells within the Pax2- or Jagged1 expressing domains. Data are presented as means +/- SEM (Standard error of the mean).
Fig. 6
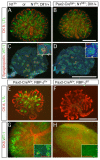
Notch1-deficient ES cells contribute to various parts of the nephron. The kidneys are harvested from E16.5 embryos (A-F) or adult (G), and subject to whole mount β-gal staining. (A, C, E) Wild type ES cells in E16.5 kidneys are found in early nephrons (blank arrowhead in A), proximal convoluted tubules (PCT, circle in C), and glomerular podocytes (blank arrowhead in E). (B, D, F, G) _Notch1_-/-; Rosa26 ES cells contribute to renal vesicles (arrow in A), S-shaped bodies (blank arrowhead in A) and podocytes in the capillary-loop stage (star in B). (D, D’) Notch1-deficient ES cells (blue) contribute to LTL-labeled PCT together with wild type cells (unstained). One LTL-labeled cross-section that composed entirely from Notch1-deficient cells is shown in D (arrowhead). (E) Glomerular podocytes develop in the absence of Notch1 (blank arrowhead). The circle indicates the capillary lumen with red blood cells in it. (G) The adult kidney is stained with LTL (brown). Some of the LTL-labeled tubules in the inner cortex are entirely derived from Notch1-/- cells (solid arrow). Blank arrowhead and sold arrowhead indicate a juxtamedullary glomerulus and a cortical glomerulus, respectively. Gray arrowhead in C, D insets mark the duct.
Fig. 7
Notch1-deficient metanephroi are phenotypically wild type. The genotypes are marked above all panels. Where indicated, floxed alleles were recombined with the Pax2-Cretg/+ strain. (A,B,E,F) Whole mount staining of LTL (green) and CK8 (red) detects extensive renal tubulogenesis. Loss of Notch1 did not alter proximal tubule formation (D) whereas loss of RBP-J resulted in loss of LTL-positive epithelial cells. Note that loss of Notch1 or RBP-J in the duct (see Notch1 staining in inset, C-D) did not prevent duct branching. (C,D,G,H) Glomerular podocytes are labeled with WT-1 (green). In (G, H) whole mount preparation is also stained with CK8 (red). WT-1High, synaptopodin-positive (red in C,D, inset in G) Glomeruli are present in wild type (C), Notch1 deficient metanephroi (D), and RBP-J heterozygotes (G) but not in RBP-J deficient metanephroi (inset in H, no WT-1 or synaptopodin) (Bar, 0.5mm).
Fig. 8
Constitutively active Notch1 promotes proximal tubule formation while inhibiting the progenitor from differentiating into podocytes and distal tubules. (A) Notch1 activation in the mesenchyme causes renal hypoplasia at E17.5. (B) The ureteric bud branches only once. Subsequent planes of sectioning are marked (a-c). CK8 is visualized using Cy3, but the image is pseudo-colored to correspond with figures Ba-Bc. (C, C’) E11.5 metanephroi from mutant (C) or wild type (C’) were cultured for 4 days, stained with LTL (green) and CK8 (red). (Ba-c) Serial sections of the mutant kidney are stained with markers for renal tubules. Arrows locate the same cells in adjacent sections.
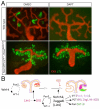
Fig. 9
(A) Ectopic over-expression of Notch1 intracellular domain is sufficient to promote proximal tubule formation in absence of γ-secretase activity. Genotypes are shown to the left of the image; DMSO-vehicle only, DAPT- γ-secretase inhibitor. (B) A schema of the proximlization pathway. After RV induction (a Wnt dependent process) Pax2 and WT-1 begin to separate into distinct expression domains and Lim1 and Dll1 define a distal domain within the RV. However, Notch2 signals are required for separation of proximal from distal fates; in their absence (or when blocked by γ-secretase inhibitors (GSI)), only distal tubules form. See text for detail.
Similar articles
- The contribution of Notch1 to nephron segmentation in the developing kidney is revealed in a sensitized Notch2 background and can be augmented by reducing Mint dosage.
Surendran K, Boyle S, Barak H, Kim M, Stomberski C, McCright B, Kopan R. Surendran K, et al. Dev Biol. 2010 Jan 15;337(2):386-95. doi: 10.1016/j.ydbio.2009.11.017. Epub 2009 Nov 13. Dev Biol. 2010. PMID: 19914235 Free PMC article. - Canonical Notch Signaling Directs the Fate of Differentiating Neurocompetent Progenitors in the Mammalian Olfactory Epithelium.
Herrick DB, Guo Z, Jang W, Schnittke N, Schwob JE. Herrick DB, et al. J Neurosci. 2018 May 23;38(21):5022-5037. doi: 10.1523/JNEUROSCI.0484-17.2018. Epub 2018 May 8. J Neurosci. 2018. PMID: 29739871 Free PMC article. - Conditional ablation of Notch signaling in pancreatic development.
Nakhai H, Siveke JT, Klein B, Mendoza-Torres L, Mazur PK, Algül H, Radtke F, Strobl L, Zimber-Strobl U, Schmid RM. Nakhai H, et al. Development. 2008 Aug;135(16):2757-65. doi: 10.1242/dev.013722. Epub 2008 Jul 17. Development. 2008. PMID: 18635610 - The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney.
Cheng HT, Kopan R. Cheng HT, et al. Kidney Int. 2005 Nov;68(5):1951-2. doi: 10.1111/j.1523-1755.2005.00627.x. Kidney Int. 2005. PMID: 16221173 Review. - Nephron progenitors in the metanephric mesenchyme.
Nishinakamura R, Uchiyama Y, Sakaguchi M, Fujimura S. Nishinakamura R, et al. Pediatr Nephrol. 2011 Sep;26(9):1463-7. doi: 10.1007/s00467-011-1806-0. Epub 2011 Feb 19. Pediatr Nephrol. 2011. PMID: 21336811 Review.
Cited by
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4 In Vivo.
Preuße K, Tveriakhina L, Schuster-Gossler K, Gaspar C, Rosa AI, Henrique D, Gossler A, Stauber M. Preuße K, et al. PLoS Genet. 2015 Jun 26;11(6):e1005328. doi: 10.1371/journal.pgen.1005328. eCollection 2015 Jun. PLoS Genet. 2015. PMID: 26114479 Free PMC article. - Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching.
Mao Y, Francis-West P, Irvine KD. Mao Y, et al. Development. 2015 Aug 1;142(15):2574-85. doi: 10.1242/dev.122630. Epub 2015 Jun 26. Development. 2015. PMID: 26116666 Free PMC article. - Role of FGFRL1 and other FGF signaling proteins in early kidney development.
Trueb B, Amann R, Gerber SD. Trueb B, et al. Cell Mol Life Sci. 2013 Jul;70(14):2505-18. doi: 10.1007/s00018-012-1189-9. Epub 2012 Oct 31. Cell Mol Life Sci. 2013. PMID: 23112089 Free PMC article. Review. - SpDamID: Marking DNA Bound by Protein Complexes Identifies Notch-Dimer Responsive Enhancers.
Hass MR, Liow HH, Chen X, Sharma A, Inoue YU, Inoue T, Reeb A, Martens A, Fulbright M, Raju S, Stevens M, Boyle S, Park JS, Weirauch MT, Brent MR, Kopan R. Hass MR, et al. Mol Cell. 2015 Aug 20;59(4):685-97. doi: 10.1016/j.molcel.2015.07.008. Epub 2015 Aug 6. Mol Cell. 2015. PMID: 26257285 Free PMC article. - Atlas of Cellular Dynamics during Zebrafish Adult Kidney Regeneration.
McCampbell KK, Springer KN, Wingert RA. McCampbell KK, et al. Stem Cells Int. 2015;2015:547636. doi: 10.1155/2015/547636. Epub 2015 May 18. Stem Cells Int. 2015. PMID: 26089919 Free PMC article.
References
- Abrahamson DR, Irwin MH, St John PL, Perry EW, Accavitti MA, Heck LW, Couchman JR. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989;109:3477–91. - PMC - PubMed
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–92. - PubMed
- Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288:F939–52. - PubMed
- Cheng H, Miner J, Lin M, Tansey MG, Roth KA, Kopan R. g-Secretase Activity is Dispensable for the Mesenchyme-to-Epithelium Transition but Required for Proximal Tubule Formation in Developing Mouse Kidney. Development. 2003;130:5031–5041. - PubMed
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development. 1998a;125:803–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 DK060319/DK/NIDDK NIH HHS/United States
- R37 DK054364/DK/NIDDK NIH HHS/United States
- DK066408/DK/NIDDK NIH HHS/United States
- R01 DK066408/DK/NIDDK NIH HHS/United States
- DK054364/DK/NIDDK NIH HHS/United States
- 5F32DK060319/DK/NIDDK NIH HHS/United States
- R01 DK054364/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous