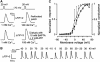Afferent neurotransmission mediated by hemichannels in mammalian taste cells - PubMed (original) (raw)
Comparative Study
Afferent neurotransmission mediated by hemichannels in mammalian taste cells
Roman A Romanov et al. EMBO J. 2007.
Abstract
In mammalian taste buds, ionotropic P2X receptors operate in gustatory nerve endings to mediate afferent inputs. Thus, ATP secretion represents a key aspect of taste transduction. Here, we characterized individual vallate taste cells electrophysiologically and assayed their secretion of ATP with a biosensor. Among electrophysiologically distinguishable taste cells, a population was found that released ATP in a manner that was Ca(2+) independent but voltage-dependent. Data from physiological and pharmacological experiments suggested that ATP was released from taste cells via specific channels, likely to be connexin or pannexin hemichannels. A small fraction of ATP-secreting taste cells responded to bitter compounds, indicating that they express taste receptors, their G-protein-coupled and downstream transduction elements. Single cell RT-PCR revealed that ATP-secreting taste cells expressed gustducin, TRPM5, PLCbeta2, multiple connexins and pannexin 1. Altogether, our data indicate that tastant-responsive taste cells release the neurotransmitter ATP via a non-exocytotic mechanism dependent upon the generation of an action potential.
Figures
Figure 1
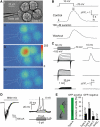
Release of ATP from taste cells. (A) Assay of ATP secretion with an ATP biosensor. (i) Simultaneous recording from the patch-clamped taste cell and COS-1 cell biosensors loaded with Fluo-4. (ii–iv) Sequential fluorescent images of the COS-1 cells in control (ii) and after depolarization of the taste cell to 10 mV (iii, iv). Color palette in (iii) shows the pixel intensity mapping (range: 0–255, 8-bit data). (B) A relative change in Fluo-4 fluorescence recorded from the upper-left COS-1 cell in (Ai) triggered by taste cell depolarization (upper inset). The images (ii–iv) in (A) were captured at the time points indicated by the letters above the upper trace. The middle and bottom traces represent responses of the same COS-1 cell in the presence of 100 μM suramin and after washout. (C) Correlations between electrophysiological characteristics of taste cells (left panels) and their ability to release ATP (right panels). Only taste cells exhibiting integral currents of type A (upper-left traces) secreted ATP upon depolarization, whereas cells with type B (middle-left traces) or type C (bottom-left traces) currents never stimulated the ATP sensor upon depolarization. The upper insets indicate command voltage used for cell identification (left panel) and for the stimulation of ATP release (right panel). (D) Left panel: a type A cell held at −70 mV generated an inward current of about 400 pA in response to the bitter mix. Right panel: type A cells are electrically excitable. Typically (_n_=14), inward currents elicited action potentials (APs) with the threshold of 10–15 pA, suggesting generator currents (left panel) to produce APs. (E) Taste cells isolated from GFP-transgenic mice (left panel). As shown in the right panel, all gustducin-positive (green) cells (_n_=48) exhibited integral currents of A type, whereas the population of gustducin-negative (dark) cells (_n_=42) contain all electrophysiologically defined subtypes. The numbers of tested cells are indicated above the bars. In all cases, currents/voltage were recorded with 140 mM KCl in the pipette and 140 mM NaCl in the bath using the perforated patch approach.
Figure 2
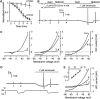
TRPM5-like cation channels operate in type A cells. (A) Evolution trace for outward currents in the presence of 5 μM U73122 (•) (_n_=7) and 15 μM U73343 (○) (_n_=4), a less potent PLC inhibitor, exhibited half-inhibition times of about 2.5 and 11 min, respectively. The VG currents were recorded from type A cells at 50 mV and normalized to the value obtained immediately before drug application. (B) Effects exerted by 5 μM U7312 and 1 μM ionomycin on the resting currents recorded at −70 mV with 140 mM NaCl or 140 mM NMDGCl in the bath (_n_=11). The short current transients were produced by cell polarization with the voltage ramp from −80 to 80 mV (1 mV/ms) to generate the I_–_V curves presented in (C). (C) Left panel: I_–_V curves generated before (1) and 4 min after (2) U73122 application. Middle panel: with VG outward currents inhibited (3 and 4), an increase in membrane permeability (5 and 6) produced by ionomycin was well resolved. Right panel: the substitution of external NaCl for NMDGCl negatively shifted the reversal potential of the ionomycin-stimulated (IS) current from −2 to −54 mV. The IS current was calculated as the difference between currents recorded before and after ionomycin application (5–4 and 6–3). (D) Upper trace: a drop in bath temperature strongly (_Q_10=4.7) suppressed the IS current. Bottom trace: bath temperature in control (24.5°C) and during application and removal of cooled bath solution. (E) I_–_V curves were generated at the time points indicated in (D) at two different temperatures before (1 and 2) and after application of 2 μM ionomycin (3 and 4). In the inset, ratio _I_t/_I_25 versus temperature; _I_t and _I_25 are IS currents at −80 mV and at temperatures t and 25°C, respectively. The line regression of the data from five different cells yielded the slope of 4.7. In (A), the recording conditions were as in Figure 1E. In (B–D), cells were dialyzed with the intracellular Cs+ solution containing 1 mM EGTA and perfused with the Na+ bath solution.
Figure 3
ATP is released from taste cells via channels. (A) ATP secretion triggered by depolarization (upper insets) was apparently independent of Ca2+. Upper traces: ATP release from the same cell was weakly dependent on extracellular Ca2+. Bottom traces: ATP secretion from the same cell was not diminished by dialysis with 10 mM BAPTA (left panel) or by the drop in extracellular Ca2+ (right panel). The patch pipette contained 140 mM CsCl, 2 mM K2ATP, and 10 mM BAPTA. (B) Responses of the ATP sensor to serial depolarization of a taste cell to specific potentials (indicated above the traces) for 5 s. (C) ATP responses (Δ) of COS-1 cells as a function of taste cell depolarization from −70 mV to indicated voltage for 5 s. The responses of the ATP sensor correlate well with the integral conductance G of taste cells (•) calculated by the equation _I_=G(_V_−_V_r), where I, V, and _V_r are sustained current, membrane voltage, and reversal potential, respectively. The data are presented as mean±s.d. (_n_=3–7). In (A (upper trace), B, C), the recording conditions were as in Figure 1E.
Figure 4
Accumulation of Lucifer Yellow (LY) by taste cells. (A) Protocol of cell loading with LY. Upper trace: taste cell polarization with time; after the depolarization to 50 mV, cells were repolarized in two steps (upper inset) to reduce the efflux of negatively charged LY through deactivating channels. Bottom trace: LY concentration in the bath. (B) Type A cell viewed in transparent light before LY application (i) and in epifluorescence during LY loading at −70 mV (ii), and after the serial depolarization (iii). The fluorescent images were captured with a 535±25 nm optical filter at the time points marked with the letters below the bottom trace in (A). (C) WC currents recorded from the cell presented in (D) before (left panel) and after (right panel) loading with LY. The recording conditions were as in Figure 1E. (D) Taste cells of the B (i) and C (ii) types did not take up LY on serial depolarization (C), as demonstrated by the fluorescent images (dark insets) captured as in (Biii). In (Bii, Ci, Cii), the images were acquired with exposition twice longer than in (Biii).
Figure 5
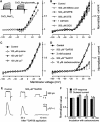
Pharmacology of outward currents and ATP release. (A) Substitution of 140 mM NaCl (•) for 140 mM Na-gluconate (○) in the bath shifted the reversal potential of sustained integral currents positively. (B–D) Effects of bath application of Cl− channel blockers (B) and hemichannel inhibitors (C, D) on sustained integral currents (_n_=3–7). As shown in (D), only octanol (3 mM) (▴) and the connexin mimetic peptide 43GAP26 (500 μM) (○) caused significant current inhibition. The I_–_V curves in the presence of 43GAP26 and carbenoxolone were generated 10 and 30 min after drug application, respectively. The data are presented as means±s.d. (E) 43GAP26 (500 μM) inhibited reciprocally both the outward currents (upper panel) and the ATP sensor responses (bottom panel) elicited by the serial depolarization of a taste cell from −70 to 10 mV. (F) Both outward currents and ATP release were weakly sensitive to 10 μM carbenoxolone. In all cases, taste cells were assayed by using the perforated patch approach with 140 mM CsCl in the recording pipette and with 140 mM NaCl in the bath.
Figure 6
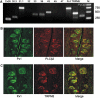
Expression of signaling and junctional proteins in taste cells. (A) Linear RNA amplification and PCR analysis of the indicated gene transcripts in a preparation of single cells of type A. The expected amplification products were obtained for Cx26, Cx30.3, Cx31.1, Cx33, Cx36, Cx43, Px1, TRPM5, and PLCβ2. Transcripts for Cx 32 (lane 4), Cx 45 (lane 8), and Cx 47 (lane 9) were not detected, although relevant products were amplified in control preparations of the brain (not shown). Molecular weight markers (M) from Gene Ruler 1-kb ladder (Fermentas). The 1.4% agarose gels were stained with ethidium bromide. (B, C) Confocal images of mouse circumvallate taste buds double labeled with antibodies against Px1 (green) and PLCβ2 or TRPM5 (red). Right panels show the merged images indicating the significant overlap in expression (yellow–orange) of Px1 with PLCβ2 and/or TRPM5 in type II taste cells.
Figure 7
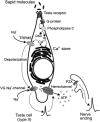
Schematic model showing the hypothetical sequence of intracellular events that are triggered by the binding of sapid molecules to taste receptors and culminate in ATP release through hemichannels.
Similar articles
- Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells.
Huang YA, Roper SD. Huang YA, et al. J Physiol. 2010 Jul 1;588(Pt 13):2343-50. doi: 10.1113/jphysiol.2010.191106. Epub 2010 May 24. J Physiol. 2010. PMID: 20498227 Free PMC article. - The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds.
Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Huang YJ, et al. Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6436-41. doi: 10.1073/pnas.0611280104. Epub 2007 Mar 26. Proc Natl Acad Sci U S A. 2007. PMID: 17389364 Free PMC article. - Knocking out P2X receptors reduces transmitter secretion in taste buds.
Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G, Chaudhari N, Finger TE, Kinnamon SC, Roper SD. Huang YA, et al. J Neurosci. 2011 Sep 21;31(38):13654-61. doi: 10.1523/JNEUROSCI.3356-11.2011. J Neurosci. 2011. PMID: 21940456 Free PMC article. - The sweet and the bitter of mammalian taste.
Scott K. Scott K. Curr Opin Neurobiol. 2004 Aug;14(4):423-7. doi: 10.1016/j.conb.2004.06.003. Curr Opin Neurobiol. 2004. PMID: 15321062 Review. - Taste receptor signalling - from tongues to lungs.
Kinnamon SC. Kinnamon SC. Acta Physiol (Oxf). 2012 Feb;204(2):158-68. doi: 10.1111/j.1748-1716.2011.02308.x. Epub 2011 May 7. Acta Physiol (Oxf). 2012. PMID: 21481196 Free PMC article. Review.
Cited by
- Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells.
Huang YA, Roper SD. Huang YA, et al. J Physiol. 2010 Jul 1;588(Pt 13):2343-50. doi: 10.1113/jphysiol.2010.191106. Epub 2010 May 24. J Physiol. 2010. PMID: 20498227 Free PMC article. - Synaptic communication and signal processing among sensory cells in taste buds.
Chaudhari N. Chaudhari N. J Physiol. 2014 Aug 15;592(16):3387-92. doi: 10.1113/jphysiol.2013.269837. Epub 2014 Mar 24. J Physiol. 2014. PMID: 24665098 Free PMC article. - Role of the ectonucleotidase NTPDase2 in taste bud function.
Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE, Kinnamon SC. Vandenbeuch A, et al. Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):14789-94. doi: 10.1073/pnas.1309468110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959882 Free PMC article. - Why do taste cells generate action potentials?
Vandenbeuch A, Kinnamon SC. Vandenbeuch A, et al. J Biol. 2009;8(4):42. doi: 10.1186/jbiol138. Epub 2009 Apr 28. J Biol. 2009. PMID: 19439032 Free PMC article. Review. - Sarco/Endoplasmic reticulum Ca2+-ATPases (SERCA) contribute to GPCR-mediated taste perception.
Iguchi N, Ohkuri T, Slack JP, Zhong P, Huang L. Iguchi N, et al. PLoS One. 2011;6(8):e23165. doi: 10.1371/journal.pone.0023165. Epub 2011 Aug 2. PLoS One. 2011. PMID: 21829714 Free PMC article.
References
- Avenet P, Lindemann B (1991) Noninvasive recording of receptor cell action potentials and sustained currents from single taste buds maintained in the tongue: the response to mucosal NaCl and amiloride. J Membr Biol 124: 33–41 - PubMed
- Bao L, Locovei S, Dahl G (2004) Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68 - PubMed
- Baryshnikov SG, Rogachevskaja OA, Kolesnikov SS (2003) Calcium signaling mediated by P2Y receptors in mouse taste cells. J Neurophysiol 90: 3283–3294 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous