MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly - PubMed (original) (raw)
Comparative Study
MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly
Cerstin Franz et al. EMBO Rep. 2007 Feb.
Abstract
The metazoan nuclear envelope (NE) breaks down and re-forms during each cell cycle. Nuclear pore complexes (NPCs), which allow nucleocytoplasmic transport during interphase, assemble into the re-forming NE at the end of mitosis. Using in vitro NE assembly, we show that the vertebrate homologue of MEL-28 (maternal effect lethal), a recently discovered NE component in Caenorhabditis elegans, functions in postmitotic NPC assembly. MEL-28 interacts with the Nup107-160 complex (Nup for nucleoporin), an important building block of the NPC, and is essential for the recruitment of the Nup107-160 complex to chromatin. We suggest that MEL-28 acts as a seeding point for NPC assembly.
Figures
Figure 1
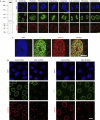
Characterization of MEL-28 in human cells. (A) Antibodies against human MEL-28 recognize a high-molecular-weight protein in HeLa nuclear extracts. Asterisks indicate a crossreacting band. (B) Immunolocalization of MEL-28 throughout the cell cycle in HeLa cells: chromatin stained with DAPI is shown in the upper row, MEL-28 (green) in the middle row and NPCs visualized with mAb414 (red) in the lower row. Scale bars, 15 μm (left column) and 5 μm (all others). (C) MEL-28 colocalizes with NPCs: a surface image of the NE from a U2OS nucleus is shown using the same colour coding as in (B). Scale bar, 8 μm. (D) RNA interference depletion of MEL-28: HeLa cells, transfected with either control or MEL-28 siRNA duplexes, were fixed after 72 h and immunostained with MEL-28 antibodies (green; left columns), LEM2 antibodies (green; right columns) or mAb414. DAPI staining is shown in blue. Scale bar, 15 μm. DAPI, 4,6-diamidino-2-phenylindole; LEM, lamina-associated polypeptide-emerin-MAN1; MEL, maternal effect lethal; NE, nuclear envelope; NPC, nuclear pore complexes; siRNA, short interfering RNA.
Figure 2
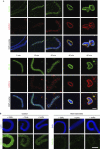
Xenopus MEL-28 is recruited early to chromatin in a Ran-dependent manner. (A) Demembranated sperm chromatin was preincubated with Xenopus egg extract for 5 min and membranes were added to the reaction. Assembly reactions were stopped by fixation at the indicated time points after the addition of cytosol and analysed by confocal microscopy after immunofluorescence using Nup160 antibodies (green, upper panel), MEL-28 antibodies (green, lower panel) or mAb414 (red). Chromatin was stained with DAPI (blue in the overlays). Scale bar, 10 μm. (B) Membrane-free cytosol was preincubated on ice for 15 min with 5 μM RanQ69L, RanT24N or buffer and then added to sperm head chromatin. The reactions were stopped by fixation after 10 min and samples were processed for immunofluorescence with MEL-28 antibodies. Non-specific binding of the antibodies to chromatin is shown after incubation with heat-inactivated extracts, which were free of the respective antigens, that is, conditions that allow only decondensation of sperm DNA. Scale bar, 10 μm. DAPI, 4,6-diamidino-2-phenylindole; mAb; monoclonal antibody; MEL, maternal effect lethal; Nup, nucleoporin.
Figure 3
Removal of MEL-28 inhibits nuclear pore complex but not nuclear envelope formation. (A) MEL-28 was depleted from the Xenopus nuclear reconstitution system by two rounds of incubation with immobilized MEL-28 antibodies: western blot analysis of mock-depleted and MEL-28-depleted extracts using the antibodies indicated on the left. (B) MEL-28-depleted extracts assemble nuclei without NPCs: nuclei were assembled for 90 min. Samples were fixed with 4% PFA and 0.5% glutaraldehyde and analysed for membrane staining (DiIC18, left column) or the presence of NPCs by immunofluorescence with mAb414. Samples in the other four columns were fixed with 4% PFA and analysed by immunofluorescence with the indicated antibodies. Chromatin was stained with DAPI (blue). Scale bars, 10 μm. (C) Transmission electron microscopy of nuclei assembled from mock-depleted (left side) or MEL-28-depleted (right side) extracts. Scale bar, 100 nm (upper images) and 2 μm (lower images). (D) Nuclei assembled in MEL-28-depleted extracts have a closed NE: an exclusion assay on chromatin incubated in RanQ69L-treated, mock-depleted or MEL-28-depleted extracts was carried out. Labelling of chromatin with Oregon green–streptavidin conjugate (green) indicates the absence of a closed NE. Scale bar, 10 μm. 1st, first round; 2nd, second round; DAPI, 4,6-diamidino-2-phenylindole; LE, Long exposure; mAb, monoclonal antibody; MEL, maternal effect lethal; NE, nuclear envelope; NPC, nuclear pore complexes; Nup, nucleoporin; PFA, paraformaldehyde.
Figure 4
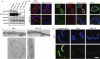
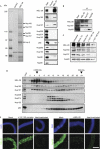
MEL-28 interacts with the Nup107–160 complex and is required for Nup107–160 recruitment to chromatin. (A) MEL-28 antibodies co-immunoprecipitate the Nup107–160 complex. The indicated proteins were identified by mass spectrometry after silver staining (left) or by western blotting with respective antisera (right) after immunoprecipitation from cytosol. Two high-molecular-weight bands that stain weakly on the silver gel represent MEL-28 (not shown). The asterisk indicates a background signal for p62. (B) Co-immunopreciptation of Nup107 and MEL-28: immunoprecipitations were performed from cytosol with antibodies against MEL-28, Nup107 or Nup205 as a control. The indicated proteins were identified by western blotting with respective antisera. (C) Depletion of the Nup107–160 complex reduces but does not fully deplete MEL-28: extracts were depleted of the Nup107–160 complex by two passages over an affinity resin. Untreated starting material, mock-depleted extracts and Nup107–160-depleted extracts after each round of depletion were analysed by western blotting with the indicated antisera. (D) Both MEL-28 and the Nup107–160 complex exist as free pools: extracts were separated on a Superose 6 gel filtration column and analysed by western blotting for the indicated proteins. Fraction 1 corresponds to the void; elution of dextran 2000 and ferritin markers for 2,000 and 550 kDa, respectively, are indicated. (E) MEL-28 is recruited to chromatin in the absence of the Nup107–160 complex, but not vice versa: sperm heads were incubated for 20 min in mock-depleted, Nup107–160-depleted, MEL-28-depleted or heat-inactivated extracts, fixed and analysed by immunofluorescence. Chromatin (upper row, blue) is stained with DAPI. MEL-28 or Nup160 (green) is shown in the lower row. Scale bar, 10 μm. 1st, first round; 2nd, second round; DAPI, 4,6-diamidino-2-phenylindole; MEL, maternal effect lethal; Nup, nucleoporin.
Similar articles
- The Role of Nucleoporin Elys in Nuclear Pore Complex Assembly and Regulation of Genome Architecture.
Shevelyov YY. Shevelyov YY. Int J Mol Sci. 2020 Dec 13;21(24):9475. doi: 10.3390/ijms21249475. Int J Mol Sci. 2020. PMID: 33322130 Free PMC article. Review. - Cell cycle-dependent differences in nuclear pore complex assembly in metazoa.
Doucet CM, Talamas JA, Hetzer MW. Doucet CM, et al. Cell. 2010 Jun 11;141(6):1030-41. doi: 10.1016/j.cell.2010.04.036. Cell. 2010. PMID: 20550937 Free PMC article. - Identification of Conserved MEL-28/ELYS Domains with Essential Roles in Nuclear Assembly and Chromosome Segregation.
Gómez-Saldivar G, Fernandez A, Hirano Y, Mauro M, Lai A, Ayuso C, Haraguchi T, Hiraoka Y, Piano F, Askjaer P. Gómez-Saldivar G, et al. PLoS Genet. 2016 Jun 24;12(6):e1006131. doi: 10.1371/journal.pgen.1006131. eCollection 2016 Jun. PLoS Genet. 2016. PMID: 27341616 Free PMC article. - The nucleoporin ELYS/Mel28 regulates nuclear envelope subdomain formation in HeLa cells.
Clever M, Funakoshi T, Mimura Y, Takagi M, Imamoto N. Clever M, et al. Nucleus. 2012 Mar 1;3(2):187-99. doi: 10.4161/nucl.19595. Epub 2012 Mar 1. Nucleus. 2012. PMID: 22555603 Free PMC article. - Nuclear pore dynamics during the cell cycle.
Imamoto N, Funakoshi T. Imamoto N, et al. Curr Opin Cell Biol. 2012 Aug;24(4):453-9. doi: 10.1016/j.ceb.2012.06.004. Epub 2012 Jul 5. Curr Opin Cell Biol. 2012. PMID: 22770730 Review.
Cited by
- Regulation of outer kinetochore assembly during meiosis I and II by CENP-A and KNL-2/M18BP1 in C. elegans oocytes.
Bellutti L, Macaisne N, El Mossadeq L, Ganeswaran T, Canman JC, Dumont J. Bellutti L, et al. Curr Biol. 2024 Nov 4;34(21):4853-4868.e6. doi: 10.1016/j.cub.2024.09.004. Epub 2024 Sep 30. Curr Biol. 2024. PMID: 39353426 - Nuclear Pores Assemble from Nucleoporin Condensates During Oogenesis.
Hampoelz B, Schwarz A, Ronchi P, Bragulat-Teixidor H, Tischer C, Gaspar I, Ephrussi A, Schwab Y, Beck M. Hampoelz B, et al. Cell. 2019 Oct 17;179(3):671-686.e17. doi: 10.1016/j.cell.2019.09.022. Cell. 2019. PMID: 31626769 Free PMC article. - The Quest for the Blueprint of the Nuclear Pore Complex.
Glavy JS. Glavy JS. Protein J. 2019 Aug;38(4):363-376. doi: 10.1007/s10930-019-09858-z. Protein J. 2019. PMID: 31410705 Free PMC article. Review. - The Nuclear Pore Complex: Birth, Life, and Death of a Cellular Behemoth.
Dultz E, Wojtynek M, Medalia O, Onischenko E. Dultz E, et al. Cells. 2022 Apr 25;11(9):1456. doi: 10.3390/cells11091456. Cells. 2022. PMID: 35563762 Free PMC article. Review. - Regulation and coordination of nuclear envelope and nuclear pore complex assembly.
Clever M, Mimura Y, Funakoshi T, Imamoto N. Clever M, et al. Nucleus. 2013 Mar-Apr;4(2):105-14. doi: 10.4161/nucl.23796. Epub 2013 Feb 14. Nucleus. 2013. PMID: 23412657 Free PMC article.
References
- Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW (2005) The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol Cell 17: 83–92 - PubMed
- Brachner A, Reipert S, Foisner R, Gotzmann J (2005) LEM2 is a novel MAN1-related inner nuclear membrane protein associated with A-type lamins. J Cell Sci 118: 5797–5810 - PubMed
- Fernandez AG, Piano F (2006) Network analysis of C. elegans early embryogenesis identifies MEL-28 as a central coordinator of chromatin maintenance and nuclear envelope function. Curr Biol 16: 1757–1763 - PubMed
- Galy V, Askjaer P, Franz C, López-Iglesias C, Mattaj IW (2006) MEL-28, a novel nuclear envelope and kinetochore protein essential for zygotic nuclear envelope assembly in C. elegans. Curr Biol 16: 1748–1756 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous