Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia - PubMed (original) (raw)
Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia
Lee J Quinton et al. J Immunol. 2007.
Abstract
Eradication of bacteria in the lower respiratory tract depends on the coordinated expression of proinflammatory cytokines and consequent neutrophilic inflammation. To determine the roles of the NF-kappaB subunit RelA in facilitating these events, we infected RelA-deficient mice (generated on a TNFR1-deficient background) with Streptococcus pneumoniae. RelA deficiency decreased cytokine expression, alveolar neutrophil emigration, and lung bacterial killing. S. pneumoniae killing was also diminished in the lungs of mice expressing a dominant-negative form of IkappaBalpha in airway epithelial cells, implicating this cell type as an important locus of NF-kappaB activation during pneumonia. To study mechanisms of epithelial RelA activation, we stimulated a murine alveolar epithelial cell line (MLE-15) with bronchoalveolar lavage fluid (BALF) harvested from mice infected with S. pneumoniae. Pneumonic BALF, but not S. pneumoniae, induced degradation of IkappaBalpha and IkappaBbeta and rapid nuclear accumulation of RelA. Moreover, BALF-induced RelA activity was completely abolished following combined but not individual neutralization of TNF and IL-1 signaling, suggesting either cytokine is sufficient and necessary for alveolar epithelial RelA activation during pneumonia. Our results demonstrate that RelA is essential for the host defense response to pneumococcus in the lungs and that RelA in airway epithelial cells is primarily activated by TNF and IL-1.
Figures
FIGURE 1
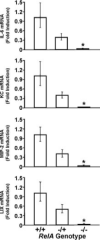
Lung cytokine mRNA expression 15 h after intratracheal SP3 (106 CFU) in presence and absence of functional RelA. All mice were on a TNFR1-deficient background, and littermates were present in each experiment for all three RelA genotypes. Cytokine mRNA levels were determined using real-time RT-PCR and normalized to the content of 18s rRNA. Data for each group were expressed as geometric means ± geometric SE (n = 6−12) of the fold induction of the values determined for RelA+/+ mice. *, p < 0.05 compared with RelA+/+ mice.
FIGURE 2
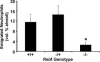
Alveolar neutrophil emigration 24 h after intratracheal SP3 (106 CFU) in presence and absence of functional RelA. All mice were on a TNFR1-deficient background, and littermates were present in each experiment for all RelA genotypes. Alveolar neutrophil counts were determined by morphometric analysis of histologic lung sections. Data for each group were expressed as means ± SE (n = 4−6) of the percentage of alveolar space occupied by neutrophils. *, p < 0.05 compared with RelA+/+ mice.
FIGURE 3
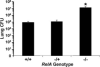
Lung bacterial killing 48 h after intratracheal SP19 (106 CFU) in the presence and absence of functional RelA. All mice were on a TNFR1-deficient background, and littermates were present in each experiment for all RelA genotypes. Viable SP19 were quantified by colony counts on 5% sheep blood agar plates. Data for each group were expressed as means ± SE (n = 6−9) total lung CFU. Statistical analyses were performed on values normalized to the inoculum in a given experiment. *, p < 0.05 compared with RelA+/+ mice.
FIGURE 4
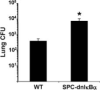
Lung bacterial killing 48 h after intratracheal SP19 (106 CFU) in the presence and absence of dnI_κ_B_α_ in airway epithelial cells. Transgenic mice overexpressed the NF-κ_B inhibitor dnI_κ_B_α under transcriptional control of the SP-C promoter. Viable SP19 were quantified by colony counts on 5% sheep blood agar plates. Data for each group were expressed as means ± SE (n = 8−11) total lung CFU. Statistical analyses were performed on values normalized to the inoculum in a given experiment. *, p < 0.05 compared with WT mice.
FIGURE 5
I_κ_B_α_ and I_κ_B_β_ degradation in MLE-15 cells stimulated with SP3 (106 CFU/ml) or BALF. BALF was collected from mice (129/Sv × C57BL/6) and pooled 0 or 15 h after intratracheal SP3 (106 CFU). MLE-15 cells were treated for 10 or 60 min with SP3 or with BALF for 10 min and then lysed for protein extractions. I_κ_B_α_ and I_κ_B_β_ levels were visualized by immunoblot, with _β_-actin serving as a loading control. The data shown represent one of three separate experiments.
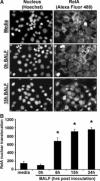
FIGURE 6
RelA nuclear translocation in MLE-15 cells 10 min after stimulation with BALF. BALF was collected from mice (129/Sv × C57BL/6) at the indicated times after intratracheal SP3 (106 CFU/ml), and MLE-15 cells were incubated with BALF from individual mice for 10 min. Scanning cytometry was used to measure cytosolic and nuclear RelA content in fixed cells. A, Representative images are shown from MLE-15 cells exposed to media, 0-h BALF, or 15-h BALF. Alexa Fluor 488 fluorescence intensity corresponds to RelA content, whereas Hoechst intensity was used as a nuclear counterstain to discriminate between cytosolic and nuclear compartments for each cell. B, RelA nuclear translocation in response to pneumonic BALF was calculated as the difference between nuclear and cytosolic Alexa Fluor 488 fluorescence intensity. Data were expressed as means ± SE of the average values obtained from BALF of individual mice (n = 6−8). Data for cells treated with media alone represented the average ± SE of data collected from 4 wells. *, p < 0.05 compared with media only treatment.
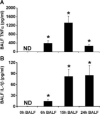
FIGURE 7
TNF-α (A) and IL-1_β_ (B) levels in BALF in response to i.t. SP3 (106 CFU). Cytokine protein levels were determined by ELISA in BALF collected from mice (129/Sv × C57BL/6) at the indicated times after i.t. SP3. Samples used for cytokine analyses were the same as those used for scanning cytometry in Fig. 6. Data for each group were expressed as means ± SE (n = 4−8) in pg/ml. ND, Not detected (below the limit of detection). For statistical analyses, the lowest standard curve values were substituted for those below the limit of detection. *, p < 0.05 compared with 0 h.
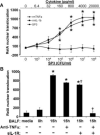
FIGURE 8
TNF-α and IL-1_β_, but not SP3, are sufficient and necessary for RelA nuclear translocation in MLE-cells. Scanning cytometry was used to determine nuclear RelA content in MLE-15 cells incubated for 10 min with cytokines, SP3, or BALF. RelA nuclear translocation was calculated as the difference between nuclear and cytosolic Alexa Fluor 488 fluorescence intensity. A, MLE-15 cells were incubated for 10 min with the indicated concentrations of cytokines or bacteria. B, BALF was collected from mice (129/Sv × C57BL/6) at the indicated times after intratracheal SP3 (106 CFU/ml), pooled, and exposed to MLE-15 cells for 10 min. Samples were supplemented with a TNF-_α_-neutralizing Ab, sIL-1RI/Fc chimera (sIL-IR), and/or the appropriate vehicle(s) for each inhibitor. Data for each group were expressed as means ± SE (n = 3) of the average values obtained from three separate experiments (samples run in triplicate for each experiment). *, p < 0.05 compared with (A) media or (B) media plus inhibitor vehicles. †, p < 0.05 compared with (B) 15-h BALF.
Similar articles
- Nuclear factor-kappaB activation in mouse lung lavage cells in response to Streptococcus pneumoniae pulmonary infection.
Amory-Rivier CF, Mohler J, Bédos JP, Azoulay-Dupuis E, Henin D, Muffat-Joly M, Carbon C, Moine P. Amory-Rivier CF, et al. Crit Care Med. 2000 Sep;28(9):3249-56. doi: 10.1097/00003246-200009000-00021. Crit Care Med. 2000. PMID: 11008989 - Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia.
Pittet LA, Quinton LJ, Yamamoto K, Robson BE, Ferrari JD, Algül H, Schmid RM, Mizgerd JP. Pittet LA, et al. Am J Respir Cell Mol Biol. 2011 Sep;45(3):573-81. doi: 10.1165/rcmb.2010-0210OC. Epub 2011 Jan 7. Am J Respir Cell Mol Biol. 2011. PMID: 21216972 Free PMC article. - Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia.
Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Jones MR, et al. J Immunol. 2005 Dec 1;175(11):7530-5. doi: 10.4049/jimmunol.175.11.7530. J Immunol. 2005. PMID: 16301661 Free PMC article. - Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia.
Yamamoto K, Ferrari JD, Cao Y, Ramirez MI, Jones MR, Quinton LJ, Mizgerd JP. Yamamoto K, et al. J Immunol. 2012 Sep 1;189(5):2450-9. doi: 10.4049/jimmunol.1200634. Epub 2012 Jul 27. J Immunol. 2012. PMID: 22844121 Free PMC article. - TNF-alpha compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia.
Rijneveld AW, Florquin S, Branger J, Speelman P, Van Deventer SJ, van der Poll T. Rijneveld AW, et al. J Immunol. 2001 Nov 1;167(9):5240-6. doi: 10.4049/jimmunol.167.9.5240. J Immunol. 2001. PMID: 11673538
Cited by
- Activation of Hepatic STAT3 Maintains Pulmonary Defense during Endotoxemia.
Hilliard KL, Allen E, Traber KE, Kim Y, Wasserman GA, Jones MR, Mizgerd JP, Quinton LJ. Hilliard KL, et al. Infect Immun. 2015 Oct;83(10):4015-27. doi: 10.1128/IAI.00464-15. Epub 2015 Jul 27. Infect Immun. 2015. PMID: 26216424 Free PMC article. - Consequences of Hypoxia for the Pulmonary Alveolar Epithelial Cell Innate Immune Response.
Sturrock A, Woller D, Freeman A, Sanders K, Paine R 3rd. Sturrock A, et al. J Immunol. 2018 Dec 1;201(11):3411-3420. doi: 10.4049/jimmunol.1701387. Epub 2018 Oct 31. J Immunol. 2018. PMID: 30381478 Free PMC article. - Mechanisms of the hepatic acute-phase response during bacterial pneumonia.
Quinton LJ, Jones MR, Robson BE, Mizgerd JP. Quinton LJ, et al. Infect Immun. 2009 Jun;77(6):2417-26. doi: 10.1128/IAI.01300-08. Epub 2009 Mar 16. Infect Immun. 2009. PMID: 19289507 Free PMC article. - Capacity of Pneumococci to Activate Macrophage Nuclear Factor κB: Influence on Necroptosis and Pneumonia Severity.
Coleman FT, Blahna MT, Kamata H, Yamamoto K, Zabinski MC, Kramnik I, Wilson AA, Kotton DN, Quinton LJ, Jones MR, Pelton SI, Mizgerd JP. Coleman FT, et al. J Infect Dis. 2017 Aug 15;216(4):425-435. doi: 10.1093/infdis/jix159. J Infect Dis. 2017. PMID: 28368460 Free PMC article. - Roles of STAT3 in protein secretion pathways during the acute-phase response.
Ahyi AN, Quinton LJ, Jones MR, Ferrari JD, Pepper-Cunningham ZA, Mella JR, Remick DG, Mizgerd JP. Ahyi AN, et al. Infect Immun. 2013 May;81(5):1644-53. doi: 10.1128/IAI.01332-12. Epub 2013 Mar 4. Infect Immun. 2013. PMID: 23460517 Free PMC article.
References
- Michaud CM, Murray CJ, Bloom BR. Burden of disease—implications for future research. J. Assoc. Med. Assoc. 2001;285:535–539. - PubMed
- Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. J. Assoc. Med. Assoc. 1999;281:61–66. - PubMed
- Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Sanchez M, Martinez JA. Severe community-acquired pneumonia: risk factors and follow-up epidemiology. Am. J. Respir. Crit. Care Med. 1999;160:923–929. - PubMed
- Garvy BA, Harmsen AG. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation. 1996;20:499–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES00002/ES/NIEHS NIH HHS/United States
- P30 ES000002/ES/NIEHS NIH HHS/United States
- R01 HL068153-05A1/HL/NHLBI NIH HHS/United States
- R01 HL079392-03/HL/NHLBI NIH HHS/United States
- T32 HL007118/HL/NHLBI NIH HHS/United States
- HL079392/HL/NHLBI NIH HHS/United States
- HL68153/HL/NHLBI NIH HHS/United States
- R01 HL068153/HL/NHLBI NIH HHS/United States
- HL07118/HL/NHLBI NIH HHS/United States
- R01 HL079392/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases