Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation - PubMed (original) (raw)
Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation
M Maalouf et al. Neuroscience. 2007.
Abstract
Dietary protocols that increase serum levels of ketones, such as calorie restriction and the ketogenic diet, offer robust protection against a multitude of acute and chronic neurological diseases. The underlying mechanisms, however, remain unclear. Previous studies have suggested that the ketogenic diet may reduce free radical levels in the brain. Thus, one possibility is that ketones may mediate neuroprotection through antioxidant activity. In the present study, we examined the effects of the ketones beta-hydroxybutyrate and acetoacetate on acutely dissociated rat neocortical neurons subjected to glutamate excitotoxicity using cellular electrophysiological and single-cell fluorescence imaging techniques. Further, we explored the effects of ketones on acutely isolated mitochondria exposed to high levels of calcium. A combination of beta-hydroxybutyrate and acetoacetate (1 mM each) decreased neuronal death and prevented changes in neuronal membrane properties induced by 10 microM glutamate. Ketones also significantly decreased mitochondrial production of reactive oxygen species and the associated excitotoxic changes by increasing NADH oxidation in the mitochondrial respiratory chain, but did not affect levels of the endogenous antioxidant glutathione. In conclusion, we demonstrate that ketones reduce glutamate-induced free radical formation by increasing the NAD+/NADH ratio and enhancing mitochondrial respiration in neocortical neurons. This mechanism may, in part, contribute to the neuroprotective activity of ketones by restoring normal bioenergetic function in the face of oxidative stress.
Figures
Figure 1
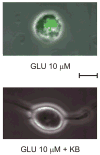
Ketones (KB) prevent neuronal injury induced by prolonged exposure to glutamate (GLU). Continuous exposure to GLU (10 μM) for 45 min resulted in the death of 50% of neurons with significant increase in propidium iodide fluorescence. A combination of 1 mM BHB and 1 mM ACA prevented neuronal death. Calibration bar = 10 μm.
Figure 2
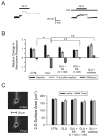
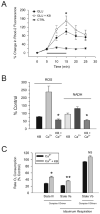
Changes in neuronal membrane properties following glutamate (GLU) excitotoxicity are prevented by ketones (KB). KB were administered as a cocktail in a 1:1 concentration ratio of BHB to ACA (either 0.1 mM or 1.0 mM each). (A) Exposure to GLU (10 μM ) for 10 min (short horizontal bar) alone resulted in an initial, small depolarization followed by a sharp, larger increase of the membrane potential (Vm) without return to baseline despite a 10 min washout period. The same protocol was used in the presence of BHB and ACA (1 mM each; long horizontal bar) but in this case, GLU induced a stable, depolarizing response with return of Vm to baseline after discontinuation of the application. (B) GLU (n = 13) significantly reduced Rm relative to control (CTRL; n = 8; p = 0.02). Following the addition of the 1 mM KB combination 20 (N = 13) or catalase 250 U/ml (n = 6), but not the 0.1 mM KB combination (n = 12), Rm was significantly higher than in cases exposed to GLU alone (p < 0.01). (C) The electrophysiological changes induced by GLU could not be attributed to morphological changes or technical difficulties. The two-dimensional surface area of the neuron exposed to GLU alone (in the absence of any fluorescent dyes) was unchanged by the experimental protocol and the position of the recording electrode was stable throughout the experiment. Neurons in all treatment groups displayed similar surface areas at the beginning and at the end of the experiments.
Figure 3
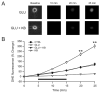
Ketones (KB) block glutamate-induced increases in superoxide radicals. (A) Exposure of neurons to glutamate (GLU; 10 μM) for 10 min led to a time-dependent increase in the fluorescence of DHE, a marker for the free radical superoxide (n = 15). In the presence of both GLU and KB (D-β-hydroxybutyrate and acetoacetate, 1 mM each), the increase in DHE fluorescence was smaller (GLU + KB; n = 16). (B) GLU (horizontal bar) provoked a time-dependent increase in DHE fluorescence that persisted after discontinuation of GLU but that was significantly reduced (p = 0.01) by KB, down to levels similar to the control group (CTRL; n = 11). The application of KB alone (KB; n = 8) significantly decreased the DHE signal below that of control experiments (p = 0.04). ** p < 0.01.
Figure 4
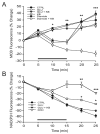
The antioxidant effects of ketones (KB) are mediated by NADH oxidation and not by an increase in reduced glutathione. (A) Acutely dissociated neurons were incubated with monochlorobimane (MCB), a fluorescent marker of reduced glutathione. The decrease in MCB fluorescence induced by 10 μM glutamate alone (GLU; n = 7) for 10 min was prevented by ketones (GLU + KB; n = 8; p < 0.01). The administration of 10 mM diamide, a specific thiol oxidant, for 10 min temporarily decreased MCB fluorescence as well (diamide; n = 10 mM; p = 0.01) but ketones did not have any effect under these conditions (diamide + KB; n = 5). Moreover, ketones did not affect baseline levels of MCB (KB; n = 5). (B) Glutamate (n = 7) increased the NAD(P)H signal relative to controls (n = 6), suggesting that the observed decrease in glutathione levels was secondary to increased production of reactive oxygen species (ROS). Ketones blocked the effect of glutamate on NAD(P)H, confirming that they decrease ROS rather an increase glutathione (n = 6). Differences were statistically significant (p = 0.01 at 20 min and 0.04 at 25 min). The effects of 1 mM potassium cyanide (KCN) are also presented for comparative purposes. Exposure to KCN for 10 min significantly increased NAD(P)H fluorescence almost to baseline levels. Horizontal bars indicate 10 min treatment periods. * p < 0.05; ** p < 0.01; *** p < 0.001.
Figure 5
Ketones (KB) reduce calcium-induced alterations in mitochondrial bioenergetics, ROS production and NAD+/NADH cycling. (A) Glutamate-mediated influx of calcium into mitochondria was not affected by ketones. Acutely dissociated neurons were incubated with the fluorescent mitochondrial calcium indicator Rhod-2. Exposure to 10 μM glutamate for 10 min (n = 5) increased mitochondrial calcium levels significantly relative to controls (n = 4). The same findings were observed when 1 mM BHB and 1 mM ACA were added (n = 4; ANOVA on ranks with Dunn’s post-hoc analyses; p < 0.05). (B) 0.5 mM calcium significantly increased mitochondrial ROS production and NADH concentrations. The addition of BHB and ACA (1 mM each) reduced both mitochondrial ROS production and NADH levels to baseline. (C) Mitochondrial respiration was assessed using a Clark-type electrode and demonstrated that calcium significantly impaired complex-I (NADH-linked) driven oxygen consumption both in response to the addition of ADP (state III respiration) or carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone [FCCP] (maximum electron transport system capacity; state Va) but did not significantly alter complex-II (FADH-linked) driven maximum electron transport system capacity (state Vb). The inclusion of 1 mM BHB and 1 mM ACA partially reversed this inhibition of respiration. * p < 0.05; ** p < 0.01.
Similar articles
- Ketone bodies are protective against oxidative stress in neocortical neurons.
Kim DY, Davis LM, Sullivan PG, Maalouf M, Simeone TA, van Brederode J, Rho JM. Kim DY, et al. J Neurochem. 2007 Jun;101(5):1316-26. doi: 10.1111/j.1471-4159.2007.04483.x. Epub 2007 Mar 30. J Neurochem. 2007. PMID: 17403035 - Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state.
Kushnareva Y, Murphy AN, Andreyev A. Kushnareva Y, et al. Biochem J. 2002 Dec 1;368(Pt 2):545-53. doi: 10.1042/BJ20021121. Biochem J. 2002. PMID: 12180906 Free PMC article. - Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs).
Rueda CB, Llorente-Folch I, Traba J, Amigo I, Gonzalez-Sanchez P, Contreras L, Juaristi I, Martinez-Valero P, Pardo B, Del Arco A, Satrustegui J. Rueda CB, et al. Biochim Biophys Acta. 2016 Aug;1857(8):1158-1166. doi: 10.1016/j.bbabio.2016.04.003. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060251 Review. - Cellular and mitochondrial changes in glutamate-induced HT4 neuronal cell death.
Tirosh O, Sen CK, Roy S, Packer L. Tirosh O, et al. Neuroscience. 2000;97(3):531-41. doi: 10.1016/s0306-4522(00)00028-2. Neuroscience. 2000. PMID: 10828535 - Mitochondrial membrane potential and the permeability transition in excitotoxicity.
Reynolds IJ. Reynolds IJ. Ann N Y Acad Sci. 1999;893:33-41. doi: 10.1111/j.1749-6632.1999.tb07816.x. Ann N Y Acad Sci. 1999. PMID: 10672228 Review.
Cited by
- Favorable Effects of Virgin Coconut Oil on Neuronal Damage and Mortality after a Stroke Incidence in the Stroke-Prone Spontaneously Hypertensive Rat.
Vitor RJS 2nd, Tochinai R, Sekizawa SI, Kuwahara M. Vitor RJS 2nd, et al. Life (Basel). 2022 Nov 11;12(11):1857. doi: 10.3390/life12111857. Life (Basel). 2022. PMID: 36430992 Free PMC article. - Infusion of sodium DL-3-ß-hydroxybutyrate decreases cerebral injury biomarkers after resuscitation in experimental cardiac arrest.
Annoni F, Su F, Peluso L, Lisi I, Caruso E, Pischiutta F, Gouvea Bogossian E, Garcia B, Njimi H, Vincent JL, Gaspard N, Ferlini L, Creteur J, Zanier ER, Taccone FS. Annoni F, et al. Crit Care. 2024 Sep 20;28(1):314. doi: 10.1186/s13054-024-05106-8. Crit Care. 2024. PMID: 39304944 Free PMC article. - Ketone bodies in epilepsy.
McNally MA, Hartman AL. McNally MA, et al. J Neurochem. 2012 Apr;121(1):28-35. doi: 10.1111/j.1471-4159.2012.07670.x. Epub 2012 Feb 7. J Neurochem. 2012. PMID: 22268909 Free PMC article. Review. - Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke.
Yin J, Han P, Tang Z, Liu Q, Shi J. Yin J, et al. J Cereb Blood Flow Metab. 2015 Nov;35(11):1783-9. doi: 10.1038/jcbfm.2015.123. Epub 2015 Jun 10. J Cereb Blood Flow Metab. 2015. PMID: 26058697 Free PMC article. - Hitting a moving target: Basic mechanisms of recovery from acquired developmental brain injury.
Giza CC, Kolb B, Harris NG, Asarnow RF, Prins ML. Giza CC, et al. Dev Neurorehabil. 2009;12(5):255-68. doi: 10.3109/17518420903087558. Dev Neurorehabil. 2009. PMID: 19956795 Free PMC article. Review.
References
- Almeida A, Heales SJ, Bolanos JP, Medina JM. Glutamate neurotoxicity is associated with nitric oxide-mediated mitochondrial dysfunction and glutathione depletion. Brain Res. 1998;790:209–216. - PubMed
- Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. - PubMed
- Atlante A, Gagliardi S, Minervini GM, Ciotti MT, Marra E, Calissano P. Glutamate neurotoxicity in rat cerebellar granule cells: a major role for xanthine oxidase in oxygen radical formation. J Neurochem. 1997;68:2038–2045. - PubMed
- Ault JG, Lawrence DA. Glutathione distribution in normal and oxidatively stressed cells. Exp Cell Res. 2003;285:9–14. - PubMed
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS048191/NS/NINDS NIH HHS/United States
- NS044846/NS/NINDS NIH HHS/United States
- R01 NS048191/NS/NINDS NIH HHS/United States
- K02 NS044846/NS/NINDS NIH HHS/United States
- R21 NS046426/NS/NINDS NIH HHS/United States
- NS046426/NS/NINDS NIH HHS/United States
- K02 NS044846-04/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources