Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration - PubMed (original) (raw)
Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration
Jian-Zhi Wang et al. Eur J Neurosci. 2007 Jan.
Abstract
Microtubule associated protein (MAP) tau is abnormally hyperphosphorylated in Alzheimer's disease (AD) and related tauopathies; in this form it is the major protein subunit of paired helical filaments (PHF)/neurofibrillary tangles. However, the nature of protein kinases and phosphatases and tau sites involved in this lesion has been elusive. We investigated self-assembly and microtubule assembly promoting activities of hyperphosphorylated tau isolated from Alzheimer disease brain cytosol, the AD abnormally hyperphosphorylated tau (AD P-tau) before and after dephosphorylation by phosphoseryl/phosphothreonyl protein phosphatase-2A (PP-2A), and then rephosphorylation by cyclic AMP-dependent protein kinase (PKA), calcium, calmodulin-dependent protein kinase II (CaMKII), glycogen synthase kinase-3beta (GSK-3beta) and cyclin-dependent protein kinase 5 (cdk5) in different kinase combinations. We found that (i) dephosphorylation of AD P-tau by PP-2A inhibits its polymerization into PHF/straight filaments (SF) and restores its binding and ability to promote assembly of tubulin into microtubules; (ii) rephosphorylation of PP-2A-dephosphorylated AD P-tau by sequential phosphorylation by PKA, CaMKII and GSK-3beta or cdk5, and as well as by cdk5 and GSK-3beta, promotes its self-assembly into tangles of PHF similar to those seen in Alzheimer brain, and (iii) phosphorylation of tau sites required for this pathology are Thr231 and Ser262, along with several sites flanking the microtubule binding repeat region. Phosphorylation of recombinant human brain tau(441) yielded similar results as the PP-2A dephosphorylated AD P-tau, except that mostly SF were formed. The conditions for the abnormal hyperphosphorylation of tau that promoted its self-assembly also induced the microtubule assembly inhibitory activity. These findings suggest that activation of PP-2A or inhibition of either both GSK-3beta and cdk5 or one of these two kinases plus PKA or CaMKII might be required to inhibit Alzheimer neurofibrillary degeneration.
Figures
Fig. 1
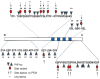
A schematic showing the AD abnormally hyperphosphorylated tau sites, the sites studied and sites found dephosphorylated by PP-2A. PP-2A effectively dephosphorylated AD P-tau at all the sites studied, but to varying degrees; dephosphorylation at Thr205, Thr212, Thr217 and Ser396 was less remarkable.
Fig. 2
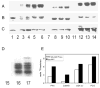
Phosphorylation of tau441 and PP-2A-AD P-tau by PKA, CaMKII, GSK-3β, and the combination of the three kinases. (A) 32P autoradiography; (B) Coomassie blue; (C) Western blots developed with tau antibody 92e to total tau; lane 1, no kinase; lanes 2, 7 and 11, with kinase but no tau; lanes 3–6, with tau and 2.5 μg/mL, 5.0 μg/mL, 10 μg/mL and 20 μg/mL of PKA; lanes 8–10, 0.5 μg/mL, 1 μg/mL, and 2 μg/mL of CaMKII, lanes 12–14, 12.5 U/mL, 25 U/mL and 50 U/mL of GSK-3β. (D) Rephosphorylation (32P autoradiography) of PP-2A-AD P-tau; lane 15, no-kinase control; lane 16, 100 U/mL GSK-3β; lane 17, 40 μg/mL, 4 μg/mL and 100 U/mL of PKA, CaMKII and GSK-3β, respectively. Phosphorylation of PP-2A-AD P-tau generated an upward shifted band. (E) Stoichiometry of the phosphorylation of tau441 by 20 μg/mL of PKA, 2 μg/mL of CaMKII, 50 U/mL of GSK-3β or all these three kinases (PCG), and phosphorylation of PP-2A-AD P-tau by double the amount of the kinases used for tau441. PKA, CaMKII and GSK-3β phosphorylated tau441 dose dependently, and phosphorylation of tau by PKA and GSK-3β led to a significant upwards mobility shift of tau in SDS-PAGE.
Fig. 3
Western blots showing site-specific dephosphorylation of AD P-tau by PP-2A and its rephosphorylation (A and B), and phosphorylation of tau441 (C and D) by PKA, CaMKII, GSK-3β and cdk5 individually and in different combinations. (A) AD P-tau isolated from AD brain (lane 1) was dephosphorylated by PP-2A (lane 2), and then rephosphorylated by PKA (lane 3), or CaMKII (lane 4), or GSK-3β (lane 5) or PKA, CaMKII and GSK-3β (lane 6); or (B) cdk5 (lane 7), PKA plus cdk5 (lane 8), or CaMKII plus cdk5 (lane 9), or cdk5 and GSK-3β (lane 10) or PKA, CaMKII and GSK-3β (lane 11). (C) Tau441 (lane 12) was phosphorylated by PKA (lane 13), or CaMKII (lane 14), or GSK-3β (lane 15), or PKA, CaMKII and GSK-3β (lane 16) or (D) cdk5 (lane 17), or PKA plus cdk5 (lane 18) or CaMKII plus cdk5 (lane 19), or cdk5 plus GSK-3β (lane 20) or PKA, CaMKII and GSK-3β (lane 21). Western blots were probed with antibodies as indicated below each blot.
Fig. 4
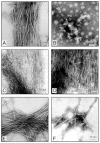
Negative stain electron microscopy of products of self assembly of AD P-tau and of this protein after its dephosphorylation with PP-2A and rephosphorylation with different combination of kinases. Incubation of untreated AD P-tau in rephosphorylation buffer for 4 h converted it into classic PHF/tangles (panel A). Dephosphorylation of AD P-tau by PP-2A abolished its self polymerization and no specific fibrous structures could be detected (panel B). Rephosphorylation of PP-2A-AD P-tau with CaMKII plus GSK-3 (panel C) induced formation of filaments with ~2.4 nm (panel C) and ~10 nm diameter (panel D). Rephosphorylation of PP-2A-AD P-tau with PKA, CaMKII and GSK-3β (panel E) or cdk5 plus GSK-3β (panel F) induced formation of ~22 nm PHF.
Fig. 5
Negative stain electron microscopy of products of self assembly of tau441 before and after phosphorylation with different combination of kinases. Phosphorylation of tau441 with PKA, CaMKII and GSK-3β (panel A), PKA, CaMKII and cdk5 (panels B and C), CAMKII plus GSK-3β (panel D) and cdk5 plus GSK-3β (panel E) induced formation of straight filaments with mostly 2.4 nm and occasionally 10 nm (panel C) diameter filaments. Incubation of tau441 without kinase showed short filaments (panel F).
Fig. 6
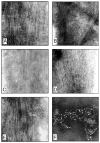
Congo-red birefringence of PP-2A-AD P-tau and tau441 after self assembly into filaments induced by phosphorylation with different kinase combinations. PP-2A-AD P-tau (panels A–C) and tau441 (panel D) were phosphorylated by PKA, CaMKII and GSK-3β (panels A and D), PKA, CaMKII and cdk5 (panel B), or by cdk5 plus GSK-3β (panel C). The products of self assembly were stained by Congo-red and birefringence was viewed and photographed by a Nikon Biophot microscope.
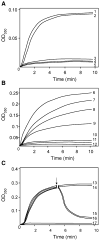
Fig. 7
In vitro assembly of microtubules measured by light scattering at 350 nm at 32 °C. (A) Microtubule assembly promoting activity of PP-2A-AD P-tau before and after rephosphorylation. AD P-tau did not promote microtubule assembly (curve 4); dephosphorylation of AD P-tau with PP-2A restored its biological activity (curve 2); rephosphorylation of PP-2A-AD P-tau by PKA, CaMKII and GSK-3β completely suppressed its ability to promote microtubule assembly (curve 3). Curve 1 shows the assembly activity of tau441 (10 μg/mL) and curve 5 of tubulin 3 mg/mL used as positive and negative controls, respectively. (B) Comparison of the microtubule assembly activity of tau441 after phosphorylation with various kinases. Phosphorylation of tau441 (30 μg/mL) with GSK-3β (curve 7), PKA (curve 8) or CaMKII (curve 9) inhibited microtubule assembly in an increasing order; the most potent inhibition was observed on phosphorylation of tau with PKA, CaMKII and GSK-3β (curve 10), which reached the same level as untreated AD P-tau (curve 11). Curve 6 is the assembly with unphosphorylated tau441 and curve 12 is with tubulin alone without addition of any tau. (C) Disruption of tau441 assembled microtubules with PP-2A-AD P-tau before and after rephosphorylation by PKA, CaMKII and GSK-3β. Microtubule assembly was initiated with tubulin (3 mg/mL) and tau441 (20 μg/mL). After 5 min (↓) of the assembly reaction, the addition of assembly buffer (100 mM MES, 1 mM EGTA, 1 mM MgCl2 and 1 mM GTP, curve 13 or PP-2A-AD P-tau in the same buffer (curve 14) did not have any significant effect on microtubule assembly. On the other hand, addition of untreated AD P-tau (curve 15) or PP-2A-AD P-tau rephosphorylated with PKA, CaMKII and GSK-3β (curve 16) disrupted the tau441 assembled microtubules. Curve 17 is a tubulin control. Rest of the details are the same as in panels A and B.
Similar articles
- Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles.
Iqbal K, Alonso AC, Gong CX, Khatoon S, Pei JJ, Wang JZ, Grundke-Iqbal I. Iqbal K, et al. J Neural Transm Suppl. 1998;53:169-80. doi: 10.1007/978-3-7091-6467-9_15. J Neural Transm Suppl. 1998. PMID: 9700655 Review. - Mechanism of neurofibrillary degeneration and pharmacologic therapeutic approach.
Iqbal K, Alonso AD, Gondal JA, Gong CX, Haque N, Khatoon S, Sengupta A, Wang JZ, Grundke-Iqbal I. Iqbal K, et al. J Neural Transm Suppl. 2000;59:213-22. doi: 10.1007/978-3-7091-6781-6_22. J Neural Transm Suppl. 2000. PMID: 10961432 Review. - Alzheimer neurofibrillary degeneration: therapeutic targets and high-throughput assays.
Iqbal K, Alonso Adel C, El-Akkad E, Gong CX, Haque N, Khatoon S, Pei JJ, Tanimukai H, Tsujio I, Wang JZ, Grundke-Iqba I. Iqbal K, et al. J Mol Neurosci. 2003;20(3):425-9. doi: 10.1385/jmn:20:3:425. J Mol Neurosci. 2003. PMID: 14501027 Review. - Pharmacological targets to inhibit Alzheimer neurofibrillary degeneration.
Iqbal K, Alonso Adel C, El-Akkad E, Gong CX, Haque N, Khatoon S, Tsujio I, Grundke-Iqbal I. Iqbal K, et al. J Neural Transm Suppl. 2002;(62):309-19. doi: 10.1007/978-3-7091-6139-5_29. J Neural Transm Suppl. 2002. PMID: 12456074 Review. - Significance and mechanism of Alzheimer neurofibrillary degeneration and therapeutic targets to inhibit this lesion.
Iqbal K, Alonso Adel C, El-Akkad E, Gong CX, Haque N, Khatoon S, Pei JJ, Tsujio I, Wang JZ, Grundke-Iqbal I. Iqbal K, et al. J Mol Neurosci. 2002 Aug-Oct;19(1-2):95-9. doi: 10.1007/s12031-002-0017-3. J Mol Neurosci. 2002. PMID: 12212801 Review.
Cited by
- Fragile X mental retardation protein: from autism to neurodegenerative disease.
Wang H. Wang H. Front Cell Neurosci. 2015 Feb 12;9:43. doi: 10.3389/fncel.2015.00043. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25729352 Free PMC article. No abstract available. - Molecular mechanisms and therapeutic potential of lithium in Alzheimer's disease: repurposing an old class of drugs.
Shen Y, Zhao M, Zhao P, Meng L, Zhang Y, Zhang G, Taishi Y, Sun L. Shen Y, et al. Front Pharmacol. 2024 Jul 11;15:1408462. doi: 10.3389/fphar.2024.1408462. eCollection 2024. Front Pharmacol. 2024. PMID: 39055498 Free PMC article. Review. - Activation of asparaginyl endopeptidase leads to Tau hyperphosphorylation in Alzheimer disease.
Basurto-Islas G, Grundke-Iqbal I, Tung YC, Liu F, Iqbal K. Basurto-Islas G, et al. J Biol Chem. 2013 Jun 14;288(24):17495-507. doi: 10.1074/jbc.M112.446070. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640887 Free PMC article. - GSKIP-Mediated Anchoring Increases Phosphorylation of Tau by PKA but Not by GSK3beta via cAMP/PKA/GSKIP/GSK3/Tau Axis Signaling in Cerebrospinal Fluid and iPS Cells in Alzheimer Disease.
Ko HJ, Chiou SJ, Wong YH, Wang YH, Lai Y, Chou CH, Wang C, Loh JK, Lieu AS, Cheng JT, Lin YT, Lu PJ, Fann MJ, Huang CF, Hong YR. Ko HJ, et al. J Clin Med. 2019 Oct 21;8(10):1751. doi: 10.3390/jcm8101751. J Clin Med. 2019. PMID: 31640277 Free PMC article. - Alzheimer's-like pathology in aging rhesus macaques: Unique opportunity to study the etiology and treatment of Alzheimer's disease.
Arnsten AFT, Datta D, Leslie S, Yang ST, Wang M, Nairn AC. Arnsten AFT, et al. Proc Natl Acad Sci U S A. 2019 Dec 26;116(52):26230-26238. doi: 10.1073/pnas.1903671116. Epub 2019 Dec 23. Proc Natl Acad Sci U S A. 2019. PMID: 31871209 Free PMC article.
References
- Alonso A, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. - PMC - PubMed
- Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000;485:87–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous