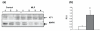Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension - PubMed (original) (raw)
Comparative Study
Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension
Irina Bogdarina et al. Circ Res. 2007.
Abstract
Hypertension is a major risk factor for cardiovascular and cerebrovascular disease. Lifelong environmental factors (eg, salt intake, obesity, alcohol) and genetic factors clearly contribute to the development of hypertension, but it has also been established that stress in utero may program the later development of the disease. This phenomenon, known as fetal programming can be modeled in a range of experimental animal models. In maternal low protein diet rat models of programming, administration of angiotensin converting enzyme inhibitors or angiotensin receptor antagonists in early life can prevent development of hypertension, thus implicating the renin-angiotensin system in this process. Here we show that in this model, expression of the AT(1b) angiotensin receptor gene in the adrenal gland is upregulated by the first week of life resulting in increased receptor protein expression consistent with the increased adrenal angiotensin responsiveness observed by others. Furthermore, we show that the proximal promoter of the AT(1b) gene in the adrenal is significantly undermethylated, and that in vitro, AT(1b) gene expression is highly dependent on promoter methylation. These data suggest a link between fetal insults to epigenetic modification of genes and the resultant alteration of gene expression in adult life leading ultimately to the development of hypertension. It seems highly probable that similar influences may be involved in the development of human hypertension.
Figures
Figure 1
Altered expression of RAS genes in MLP offspring Real-time RT-PCR was used to quantitate angiotensinogen, renin, ACE and the AT1a, AT1b and AT2 angiotensin receptor mRNAs in various control and MLP offspring rat tissues. Notable shifts in expression at either 1 or 12 weeks of age, or both, were demonstrated (a) for angiotensinogen in liver, (b) renin in kidney, and (d, e and f) the three angiotensin receptors in the adrenal. Note the different range of the y axis scale. No changes in ACE expression were apparent (c). Data are expressed as RNA copy number per μg of total RNA. White columns, control animals; black columns, MLP animals. *, p<0.05; **, p<0. 01; ***, p<0.001.
Figure 2
(a) Immunoblot of AT1a and b receptor in whole adrenal from control and MLP rats at 12 weeks age. Total MAPK is used as a loading control. (b) Densitometric quantitationof (a), corrected for protein loading and mean values + SD for 3 control (white bar) and 4 MLP adrenals (shaded bar) are shown. *, p<0.05
Figure 3
Adrenal histology from control (a) and MLP rat (b) at 10X magnification. ZG = zona glomerulosa; ZFR = zona fasciculata and reticularis. Scale bar represents 100 μm.
Figure 4
Methylation analysis of the AT1b promoter. (a) Structure of the At1b proximal promoter region showing location of putative transcription start site, consensus sequences for potential transcription factors and CpG methylation sites (open circles) analysed in this study numbered 1 - 3. Inr = Initiator element (b) Methylation of adrenal capsule AT1b gene in control and MLP animals. ○ = unmethylated; ● = methylated. One male and one female from each group had adrenal capsules harvested and used in this analysis. (c) Expression of AT1b mRNA in these same adrenal capsule samples expressed relative to 18S RNA. Control rats – white bar; MLP rats – shaded bar. ***, p<0.0001. (d) Methylation of AT1b gene proximal promoter in individual adrenals from six different animals in each group at 4 weeks of age. Each column represents an individual animal’s adrenal in which methylation status is determined at each of the three sites of interest. Fifteen clones were examined from each adrenal.
Figure 5
Influence of methylation on the AT1b promoter. Luciferase activity of AT1b-luc transiently transfected into mouse Y1 cells in the presence or absence of methylation of either one or six CpG sites in the proximal promoter using Hha1 methylase (Hha1) or Sss1 methylase (Sss1) respectively. Mutagenesis of the 3 proximal sites (1 – 3) to render them non-methylatable markedly reduces promoter activity, and methylation of the remaining promoter makes no further contribution to reduced activity. Mutagenesis of individual CpG sites in the proximal promoter reveals the considerable impact of site 3. Mean luciferase activity is expressed relative to co-transfected renilla luciferase activity (± SEM). n = 6. ○ = unmethylated site, ● = methylated site, X = mutagenised site.
Comment in
- Developmental programming through epigenetic changes.
Nuyt AM, Szyf M. Nuyt AM, et al. Circ Res. 2007 Mar 2;100(4):452-5. doi: 10.1161/01.RES.0000260292.95612.ac. Circ Res. 2007. PMID: 17332436 No abstract available.
Similar articles
- Brain renin-angiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy.
Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD. Goyal R, et al. Reprod Sci. 2010 Mar;17(3):227-38. doi: 10.1177/1933719109351935. Epub 2009 Nov 18. Reprod Sci. 2010. PMID: 19923380 - Genetic low nephron number hypertension is associated with altered expression of key components of the renin-angiotensin system during nephrogenesis.
Faensen AL, von Trebra MW, Freese F, Kreutz R, Bamberg C, Hinkson L, Rothermund L. Faensen AL, et al. J Perinat Med. 2016 Aug 1;44(6):705-9. doi: 10.1515/jpm-2015-0159. J Perinat Med. 2016. PMID: 26677883 - Glucocorticoid effects on the programming of AT1b angiotensin receptor gene methylation and expression in the rat.
Bogdarina I, Haase A, Langley-Evans S, Clark AJ. Bogdarina I, et al. PLoS One. 2010 Feb 16;5(2):e9237. doi: 10.1371/journal.pone.0009237. PLoS One. 2010. PMID: 20169056 Free PMC article. - Targeting the Renin-Angiotensin-Aldosterone System to Prevent Hypertension and Kidney Disease of Developmental Origins.
Hsu CN, Tain YL. Hsu CN, et al. Int J Mol Sci. 2021 Feb 25;22(5):2298. doi: 10.3390/ijms22052298. Int J Mol Sci. 2021. PMID: 33669059 Free PMC article. Review. - Transgenic rats: tools to study the function of the renin-angiotensin system.
Bader M, Ganten D. Bader M, et al. Clin Exp Pharmacol Physiol Suppl. 1996;3:S81-7. Clin Exp Pharmacol Physiol Suppl. 1996. PMID: 8993844 Review.
Cited by
- Metabolic syndrome: Nutri-epigenetic cause or consequence?
Silva-Ochoa AD, Velasteguí E, Falconí IB, García-Solorzano VI, Rendón-Riofrio A, Sanguña-Soliz GA, Vanden Berghe W, Orellana-Manzano A. Silva-Ochoa AD, et al. Heliyon. 2023 Oct 17;9(11):e21106. doi: 10.1016/j.heliyon.2023.e21106. eCollection 2023 Nov. Heliyon. 2023. PMID: 37954272 Free PMC article. Review. - Chemical Communication between Heart Cells is Disrupted by Intracellular Renin and Angiotensin II: Implications for Heart Development and Disease.
De Mello WC. De Mello WC. Front Endocrinol (Lausanne). 2015 May 19;6:72. doi: 10.3389/fendo.2015.00072. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26042086 Free PMC article. Review. - Epigenetic programming and risk: the birthplace of cardiovascular disease?
Vinci MC, Polvani G, Pesce M. Vinci MC, et al. Stem Cell Rev Rep. 2013 Jun;9(3):241-53. doi: 10.1007/s12015-012-9398-z. Stem Cell Rev Rep. 2013. PMID: 22773406 Review. - Antenatal maternal low protein diet: ACE-2 in the mouse lung and sexually dimorphic programming of hypertension.
Goyal R, Van-Wickle J, Goyal D, Longo LD. Goyal R, et al. BMC Physiol. 2015 May 14;15:2. doi: 10.1186/s12899-015-0016-6. BMC Physiol. 2015. PMID: 25971747 Free PMC article. - Differential expression and DNA methylation of angiotensin type 1A receptors in vascular tissues during genetic hypertension development.
Pei F, Wang X, Yue R, Chen C, Huang J, Huang J, Li X, Zeng C. Pei F, et al. Mol Cell Biochem. 2015 Apr;402(1-2):1-8. doi: 10.1007/s11010-014-2295-9. Epub 2015 Jan 18. Mol Cell Biochem. 2015. PMID: 25596947
References
- He FJ, MacGregor GA. Cost of poor blood pressure control in the UK: 62,000 unnecessary deaths per year. J Hum Hypertens. 2003;17:455–7. - PubMed
- Staessen JA, Wang J, Bianchi G, Birkenhager WH. Essential hypertension. Lancet. 2003;361:1629–41. - PubMed
- Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103–21. - PubMed
- Langley-Evans SC. Critical differences between two low protein diet protocols in the programming of hypertension in the rat. Int J Food Sci Nutr. 2000;51:11–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical