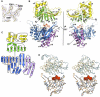Hexameric structures of the archaeal secretion ATPase GspE and implications for a universal secretion mechanism - PubMed (original) (raw)
Hexameric structures of the archaeal secretion ATPase GspE and implications for a universal secretion mechanism
Atsushi Yamagata et al. EMBO J. 2007.
Abstract
The secretion superfamily ATPases are conserved motors in key microbial membrane transport and filament assembly machineries, including bacterial type II and IV secretion, type IV pilus assembly, natural competence, and archaeal flagellae assembly. We report here crystal structures and small angle X-ray scattering (SAXS) solution analyses of the Archaeoglobus fulgidus secretion superfamily ATPase, afGspE. AfGspE structures in complex with ATP analogue AMP-PNP and Mg(2+) reveal for the first time, alternating open and closed subunit conformations within a hexameric ring. The closed-form active site with bound Mg(2+) evidently reveals the catalytically active conformation. Furthermore, nucleotide binding results and SAXS analyses of ADP, ATPgammaS, ADP-Vi, and AMP-PNP-bound states in solution showed that asymmetric assembly involves ADP binding, but clamped closed conformations depend on both ATP gamma-phosphate and Mg(2+) plus the conserved motifs, arginine fingers, and subdomains of the secretion ATPase superfamily. Moreover, protruding N-terminal domain shifts caused by the closed conformation suggest a unified piston-like, push-pull mechanism for ATP hydrolysis-dependent conformational changes, suitable to drive diverse microbial secretion and assembly processes by a universal mechanism.
Figures
Figure 1
AfGspE subunit structure. (A) Experimental electron density (contoured at 1σ) overlaid with the final refined model. (B) AfGspE fold as ribbons (molecule B) with subdomains N1 (yellow), N2 (green), C1 (blue), and C2 (magenta) plus bound AMP-PNP (red sticks), and P-loop (orange, center). (C) Topology schematic, (D) Cα stereo diagram of two superimposed afGspE subunit structures with bound AMP-PNP (red, CPK spheres). The open form (molecule A, orange) needs NTD rotations to yield the closed form (molecule B, blue).
Figure 2
Sequence conservation, secondary structure and residue function. AfGspE sequence aligned with H. salinuram FlaI, and M. voltae FlaI reveals identical (white letters in red box) and homologous residues (red letters in blue box) with secondary structure helices (bars) and strands (arrows) colored as in Figure 1B. Conserved Walker motifs A (orange) and B (green) along with Asp (pink) and His (yellow) box motifs are labeled in boxes. Dual arginine fingers (Arg208 and 227) are highlighted by magenta boxes. Also shown as largely conserved are the residues binding AMP-PNP (red reverse triangles) and in subunit-subunit interactions (blue triangles).
Figure 3
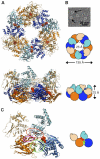
Hexameric ring structure, assembly, AMP-PNP binding, alternating subunit conformations, and subunit interactions. (A) AfGspE hexamer assembly and fold shown as ribbons and as schematic shapes viewed from top and side. Bound AMP-PNP (red, CPK spheres) in closed form molecules (blue, light blue NTD) and alternate open form molecules (orange, light orange NTD). (B) Electron micrograph of negatively stained AfGspE proteins with AMP-PNP. Ring structures are indicated by black arrows. (C) Subunit-subunit interactions with domain colors as in A, and encircled residues for N2:C1 (red), for C1:C1 (green), and N1:C2 (magenta) interactions.
Figure 4
The nucleotide binding site. Left: F o –F c difference Fourier map (contoured at 3σ) was calculated with the protein residues and superimposed onto the refined structure with AMP-PNP (purple), P-loop residues (red), Glu298 and 341 (green) in Walker B motif. The active site residues are shown in sticks. Right: nucleotide binding site showing stick models (blue nitrogens and red oxygens) and Mg2+ (gray sphere). (A) The nucleotide binding site in molecule A (open form). (B) The nucleotide binding site in molecule B (closed form). Dual arginine fingers (Arg208 and 227, red) interact with AMP-PNP γ-phosphate (dotted lines) and Glu298/341 face Mg2+ ion. (C) Phosphate binding site in closed form (molecule B). Arg227 and 408 conformational changes from the AMP-PNP bound form (light magenta) to phosphate bound form are indicated by arrows.
Figure 5
Secretion ATPase structural comparisons, subdomains, and a conserved functional core. (A) Schematic view of the three secretion ATPase structures. (B) Overall structures for HP0525 (left), afGspE (center), and EpsE (right) shown as ribbons with bound nucleotide (red sticks). Similar N2 (green) and C1 (blue) subdomains form the conserved core flanked by variable HP0525 N-terminal helix (gray), afGspE N1 (yellow) and C2 (magenta), and EpsE Zn2+ (gray sphere) region of the CM subdomain (light orange) and C2 (orange). (C) Structural comparison between afGspE (ribbons colored as in A) and HP0525 (gray) hexamers with pink HP0525 αH-I insertions. (D) Close up comparison of the closed site structure for HP0525 αH-I insertions (pink), afGspE α12–13 (blue), and EpsE (cyan). Colored residues kink the loop between helices in afGspE (red) and EpsE (yellow).
Figure 6
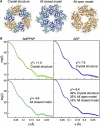
AfGspE hexamer structures in solution by SAXS. (A) Hexameric crystal structure (left), all-closed hexamer model (center), and all-open hexamer model (right). (B) X-ray scattering of the AMP-PNP bound (green) and ADP bound (blue) hexamers compared to profiles calculated from the crystal structure (red line) (top, left and right), the all-closed model (bottom left, blue line) and the mixture of crystal structure, all open hexamer, and all closed hexamer models (bottom right, orange line). The scattering data includes the error bars (gray), however, as the error is about ∼4% even in the highest angle region, it is almost invisible.
Figure 7
Nucleotide binding and the catalytic mechanism of afGspE. (A) The titration of fluorescent TNP-ATP (closed circles) and TNP-ADP (closed triangles) against the fixed afGspE concentration (0.94 μM, dotted line) is shown. The titration of TNP-ATP against the mutant afGspE K273A is shown by open circles. (B) Proposed catalytic cycle with experimentally defined open–closed conformational changes upon ATP binding and hydrolysis. In the flexible apo form (top left), the NTD (light blue) and CTD (blue) are in equilibrium between open and closed conformations. The more solvent-accessible open conformation (top right) favors ATP (red nucleotide and yellow phosphates) binding. The interaction between the dual arginine clamps (red tubes and blue nitrogen atoms) and the ATP g-phosphate locks the catalytically active closed conformation (middle right), and the resulting g-phosphate shifts allow Mg2+ (magenta sphere) to interact with the two conserved Glu residues (green tubes, red oxygen atoms). The g-phosphate release after ATP hydrolysis unlocks the closed form and accelerates the conformational change to the open form (middle left), suitable for ADP release. The primarily rigid-body movement of the top domain shown here prompts our proposal of a universal piston-like push-pull mechanism for the secretion superfamily ATPases, suitable to drive diverse microbial secretion and assembly processes. The symmetrical closed hexamer, as induced by non-hydrolysable ATP analogs such as AMP-PNP, and the asymmetrical hexamer in the ADP-bound state are shown at the bottom.
Similar articles
- Conformations of the apo-, substrate-bound and phosphate-bound ATP-binding domain of the Cu(II) ATPase CopB illustrate coupling of domain movement to the catalytic cycle.
Jayakanthan S, Roberts SA, Weichsel A, Argüello JM, McEvoy MM. Jayakanthan S, et al. Biosci Rep. 2012 Oct;32(5):443-53. doi: 10.1042/BSR20120048. Biosci Rep. 2012. PMID: 22663904 Free PMC article. - Insights into FlaI functions in archaeal motor assembly and motility from structures, conformations, and genetics.
Reindl S, Ghosh A, Williams GJ, Lassak K, Neiner T, Henche AL, Albers SV, Tainer JA. Reindl S, et al. Mol Cell. 2013 Mar 28;49(6):1069-82. doi: 10.1016/j.molcel.2013.01.014. Epub 2013 Feb 14. Mol Cell. 2013. PMID: 23416110 Free PMC article. - Modulation and Functional Role of the Orientations of the N- and P-Domains of Cu+ -Transporting ATPase along the Ion Transport Cycle.
Meng D, Bruschweiler-Li L, Zhang F, Brüschweiler R. Meng D, et al. Biochemistry. 2015 Aug 18;54(32):5095-102. doi: 10.1021/acs.biochem.5b00420. Epub 2015 Aug 5. Biochemistry. 2015. PMID: 26196187 - Mutation of a key residue in the type II secretion system ATPase uncouples ATP hydrolysis from protein translocation.
Shiue SJ, Chien IL, Chan NL, Leu WM, Hu NT. Shiue SJ, et al. Mol Microbiol. 2007 Jul;65(2):401-12. doi: 10.1111/j.1365-2958.2007.05795.x. Mol Microbiol. 2007. PMID: 17630971 - Evolution of P-type ATPases.
Palmgren MG, Axelsen KB. Palmgren MG, et al. Biochim Biophys Acta. 1998 Jun 10;1365(1-2):37-45. doi: 10.1016/s0005-2728(98)00041-3. Biochim Biophys Acta. 1998. PMID: 9693719 Review. No abstract available.
Cited by
- High-throughput SAXS for the characterization of biomolecules in solution: a practical approach.
Dyer KN, Hammel M, Rambo RP, Tsutakawa SE, Rodic I, Classen S, Tainer JA, Hura GL. Dyer KN, et al. Methods Mol Biol. 2014;1091:245-58. doi: 10.1007/978-1-62703-691-7_18. Methods Mol Biol. 2014. PMID: 24203338 Free PMC article. - The molecular mechanism of the type IVa pilus motors.
McCallum M, Tammam S, Khan A, Burrows LL, Howell PL. McCallum M, et al. Nat Commun. 2017 May 5;8:15091. doi: 10.1038/ncomms15091. Nat Commun. 2017. PMID: 28474682 Free PMC article. - Oligomerization of EpsE coordinates residues from multiple subunits to facilitate ATPase activity.
Patrick M, Korotkov KV, Hol WG, Sandkvist M. Patrick M, et al. J Biol Chem. 2011 Mar 25;286(12):10378-86. doi: 10.1074/jbc.M110.167031. Epub 2011 Jan 5. J Biol Chem. 2011. PMID: 21209100 Free PMC article. - PilB and PilT are ATPases acting antagonistically in type IV pilus function in Myxococcus xanthus.
Jakovljevic V, Leonardy S, Hoppert M, Søgaard-Andersen L. Jakovljevic V, et al. J Bacteriol. 2008 Apr;190(7):2411-21. doi: 10.1128/JB.01793-07. Epub 2008 Jan 25. J Bacteriol. 2008. PMID: 18223089 Free PMC article. - The dimer formed by the periplasmic domain of EpsL from the Type 2 Secretion System of Vibrio parahaemolyticus.
Abendroth J, Kreger AC, Hol WG. Abendroth J, et al. J Struct Biol. 2009 Nov;168(2):313-22. doi: 10.1016/j.jsb.2009.07.022. Epub 2009 Jul 29. J Struct Biol. 2009. PMID: 19646531 Free PMC article.
References
- Abendroth J, Murphy P, Sandkvist M, Bagdasarian M, Hol WG (2005) The X-ray structure of the type II secretion system complex formed by the N-terminal domain of EpsE and the cytoplasmic domain of EpsL of Vibrio cholerae. J Mol Biol 348: 845–855 - PubMed
- Abrahams JP, Leslie AG, Lutter R, Walker JE (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628 - PubMed
- Albers SV, Driessen AJ (2005) Analysis of ATPases of putative secretion operons in the thermoacidophilic archaeon Sulfolobus solfataricus. Microbiology 151: 763–773 - PubMed
- Albers SV, Driessen AM (2002) Signal peptides of secreted proteins of the archaeon Sulfolobus solfataricus: a genomic survey. Arch Microbiol 177: 209–216 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous