A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials - PubMed (original) (raw)
. 2007 Feb 21;55(4):1084-96.
doi: 10.1021/jf062431s. Epub 2007 Jan 27.
Affiliations
- PMID: 17256956
- PMCID: PMC3762687
- DOI: 10.1021/jf062431s
A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials
Long-Ze Lin et al. J Agric Food Chem. 2007.
Abstract
A screening method was developed for the systematic identification of glycosylated flavonoids and other phenolic compounds in plant food materials based on an initial, standard analytical method. This approach applies the same analytical scheme (aqueous methanol extraction, reverse phase liquid chromatographic separation, and diode array and mass spectrometric detection) to every sample and standard. This standard approach allows the cross-comparison of compounds in samples, standards, and plant materials previously identified in the published literature. Thus, every analysis contributes to a growing library of data for retention times and UV/vis and mass spectra. Without authentic standards, this method provides provisional identification of the phenolic compounds: identification of flavonoid backbones, phenolic acids, saccharides, and acyls but not the positions of the linkages between these subclasses. With standards, this method provides positive identification of the full compound: identification of subclasses and linkages. The utility of the screening method is demonstrated in this study by the identification of 78 phenolic compounds in cranberry, elder flower, Fuji apple peel, navel orange peel, and soybean seed.
Figures
Figure 1
Structures of phenolic compounds analyzed.
Figure 2
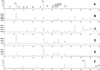
LC chromatograms of elder flower extract: (A) UV absorption at 350 nm, (B) TIC for PI100, (C) TIC for PI250, (D) TIC for NI100, (E) TIC for NI250, and (F) UV absorption at 350 nm of acid-hydrolyzed elder flower extract.
Figure 3
UV/vis absorption spectra: (A) 1, quercetin 3-_O_-galactoside (flavonol); 2, sinengetin (flavone); 3, cyanidin 3-_O_-galactoside (anthocyanin); and 4, chlorogenic acid. (B) 1, hesperidin (flavanone); 2, epicatechin (flavanol); 3, genistin (isoflavone); and 4, phloridzin (dihydrochalcone).
Figure 4
LC chromatograms with UV absorption: (A) navel orange peel (350 nm), (B) soybean seeds (270 nm), (C) Fuji apple peel (270 nm), (D) cranberry (270 nm), (E) Fuji apple peel (520 nm), and (F) cranberry (520 nm).
Figure 5
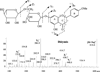
PI250 mass spectrum of peak O2, diosmetin 6,8-di-_C_-glucoside, and the related fragmentation scheme.
Figure 6
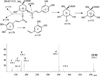
PI250 mass spectrum of peak O12, didymin, and the related fragmentation scheme.
Figure 7
NI250 mass spectra of peak E15, 4,5-dicaffeyolquinic acid, and the related fragmentation scheme.
Figure 8
LC chromatograms (350 nm) for (A) the hydroxycinnamates of navel orange peel and (B) the alkaline-hydrolyzed extract of navel orange peel.
Similar articles
- Analysis of antioxidant compounds in sweet orange peel by HPLC-diode array detection-electrospray ionization mass spectrometry.
Anagnostopoulou MA, Kefalas P, Kokkalou E, Assimopoulou AN, Papageorgiou VP. Anagnostopoulou MA, et al. Biomed Chromatogr. 2005 Mar;19(2):138-48. doi: 10.1002/bmc.430. Biomed Chromatogr. 2005. PMID: 15515108 - Analyzing cranberry bioactive compounds.
Côté J, Caillet S, Doyon G, Sylvain JF, Lacroix M. Côté J, et al. Crit Rev Food Sci Nutr. 2010 Oct;50(9):872-88. doi: 10.1080/10408390903042069. Crit Rev Food Sci Nutr. 2010. PMID: 20924868 - Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: application to different Italian varieties.
Russo M, Fanali C, Tripodo G, Dugo P, Muleo R, Dugo L, De Gara L, Mondello L. Russo M, et al. Anal Bioanal Chem. 2018 Jun;410(15):3507-3520. doi: 10.1007/s00216-018-0854-8. Epub 2018 Jan 19. Anal Bioanal Chem. 2018. PMID: 29350256 - Phenolic composition and antioxidant potential of grain legume seeds: A review.
Singh B, Singh JP, Kaur A, Singh N. Singh B, et al. Food Res Int. 2017 Nov;101:1-16. doi: 10.1016/j.foodres.2017.09.026. Epub 2017 Sep 9. Food Res Int. 2017. PMID: 28941672 Review.
Cited by
- Unmasking the Antifungal Activity of Anacardium occidentale Leaf Extract against Candida albicans.
Quejada LF, Hernandez AX, Chitiva LC, Bravo-Chaucanés CP, Vargas-Casanova Y, Faria RX, Costa GM, Parra-Giraldo CM. Quejada LF, et al. J Fungi (Basel). 2024 Jun 29;10(7):464. doi: 10.3390/jof10070464. J Fungi (Basel). 2024. PMID: 39057348 Free PMC article. - First Chemical Profile Analysis of Acacia Pods.
Pedro SI, Fernandes TA, Luís Â, Antunes AMM, Gonçalves JC, Gominho J, Gallardo E, Anjos O. Pedro SI, et al. Plants (Basel). 2023 Oct 5;12(19):3486. doi: 10.3390/plants12193486. Plants (Basel). 2023. PMID: 37836226 Free PMC article. - Comparative flavonoid profile of orange (Citrus sinensis) flavedo and albedo extracted by conventional and emerging techniques using UPLC-IMS-MS, chemometrics and antioxidant effects.
Afifi SM, Gök R, Eikenberg I, Krygier D, Rottmann E, Stübler AS, Aganovic K, Hillebrand S, Esatbeyoglu T. Afifi SM, et al. Front Nutr. 2023 Jun 6;10:1158473. doi: 10.3389/fnut.2023.1158473. eCollection 2023. Front Nutr. 2023. PMID: 37346911 Free PMC article. - Interactions of Natural Flavones with Iron Are Affected by 7-_O_-Glycosylation, but Not by Additional 6″-_O-_Acylation.
Bijlsma J, de Bruijn WJC, Koppelaar J, Sanders MG, Velikov KP, Vincken JP. Bijlsma J, et al. ACS Food Sci Technol. 2023 May 2;3(6):1111-1121. doi: 10.1021/acsfoodscitech.3c00112. eCollection 2023 Jun 16. ACS Food Sci Technol. 2023. PMID: 37342238 Free PMC article. - Comprehensive analysis of phenolics compounds in citrus fruits peels by UPLC-PDA and UPLC-Q/TOF MS using a fused-core column.
Sanches VL, Cunha TA, Viganó J, de Souza Mesquita LM, Faccioli LH, Breitkreitz MC, Rostagno MA. Sanches VL, et al. Food Chem X. 2022 Feb 22;14:100262. doi: 10.1016/j.fochx.2022.100262. eCollection 2022 Jun 30. Food Chem X. 2022. PMID: 35243328 Free PMC article.
References
- Hertog MGL, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic BS, Toshima H, Feskins EJM, Holman PCH, Katan MB. Flavonid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995;155:381–386. - PubMed
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996;96:1027–1039. - PubMed
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez C. Bioavailability and bioefficacy of polyphenols in humans. Am. J. Clin. Nutr. 2004;79:727–747. - PubMed
- Pennington JAT. Food composition databases for bioactive components. J. Food Compos. Anal. 2002;15:419–434.
- Beecher GR. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003;133(Suppl.):3048S–3054S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources