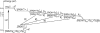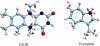Magnetic field effects in Arabidopsis thaliana cryptochrome-1 - PubMed (original) (raw)
Magnetic field effects in Arabidopsis thaliana cryptochrome-1
Ilia A Solov'yov et al. Biophys J. 2007.
Abstract
The ability of some animals, most notably migratory birds, to sense magnetic fields is still poorly understood. It has been suggested that this "magnetic sense" may be mediated by the blue light receptor protein cryptochrome, which is known to be localized in the retinas of migratory birds. Cryptochromes are a class of photoreceptor signaling proteins that are found in a wide variety of organisms and that primarily perform regulatory functions, such as the entrainment of circadian rhythm in mammals and the inhibition of hypocotyl growth in plants. Recent experiments have shown that the activity of cryptochrome-1 in Arabidopsis thaliana is enhanced by the presence of a weak external magnetic field, confirming the ability of cryptochrome to mediate magnetic field responses. Cryptochrome's signaling is tied to the photoreduction of an internally bound chromophore, flavin adenine dinucleotide. The spin chemistry of this photoreduction process, which involves electron transfer from a chain of three tryptophans, can be modulated by the presence of a magnetic field in an effect known as the radical-pair mechanism. Here we present and analyze a model of the flavin-adenine-dinucleotide-tryptophan chain system that incorporates realistic hyperfine coupling constants and reaction rate constants. Our calculations show that the radical-pair mechanism in cryptochrome can produce an increase in the protein's signaling activity of approximately 10% for magnetic fields on the order of 5 G, which is consistent with experimental results. These calculations, in view of the similarity between bird and plant cryptochromes, provide further support for a cryptochrome-based model of avian magnetoreception.
Figures
FIGURE 1
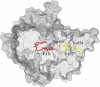
FAD cofactor and tryptophan chain in Arabidopsis thaliana cryptochrome-1. Cryptochrome is in its signaling state when the FAD cofactor is in the semireduced FADH state. The signaling state is achieved through photoreduction via a chain of three tryptophans (Trp-400, Trp-377, and Trp-324) that bridge the space between FADH and the surface of the protein, followed by deprotonation of Trp-324 to Trp-324dep.
FIGURE 2
Schematic presentation of the radical-pair reaction pathway in cryptochrome. After the flavin cofactor in its fully oxidized form, FAD, is excited by a blue photon (FAD → FAD*) and subsequently protonated (FAD* → (FADH+)*), an electron jumps from the nearby Trp-400 to FADH+, creating a radical-pair (FADH + Trp-400+) state. Electron transfer from Trp-377 to Trp-400 and from Trp-324 to Trp-377 follows, creating the radical-pair state FADH + Trp-377+ and then FADH + Trp-324+. For each radical-pair state, the spins of the unpaired electrons are in either the singlet or triplet state, as denoted by 1[⋯] or 3[⋯], respectively. Electron back-transfer, the effect of which is to quench the cryptochrome signaling state, is possible only when the two unpaired electron spins of one of the three possible radical-pair states form a singlet state 1[⋯]. If Trp-324+ becomes deprotonated (Trp-324+ → Trp-324dep), electron back-transfer FADH → Trp-324dep is impeded, and cryptochrome is stabilized in its signaling state, FADH + Trp-324dep. Transitions between the three radical-pair states, i.e., 1,3[FADH + Trp-400+], 1, 3[FADH + Trp-377+], and 1, 3[FADH + Trp-324+], are governed by the rate constant _k_et and correspond to an electron jumping between tryptophans in the direction opposite to that of the arrows shown (arrows show electron hole transfer). Electron back-transfer from FADH to one of the tryptophans is governed by the rate constant _k_b and deprotonation of the third tryptophan by the rate constant _k_d. The steps denoted by rate constants (_k_1)′ and (_k_2)′ correspond to reverse electron transfer in the tryptophan chain and are neglected in our description.
FIGURE 3
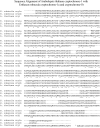
BLAST sequence alignment between Erithacus rubecula (European robin) and Arabidopsis thaliana (mouse-ear cress) cryptochromes. The alignment shows a high similarity between the bird and plant cryptochromes. Erithacus rubecula cryptochrome-1a gives an expect value of 3 × 10−38 and cryptochrome-1b gives an expect value of 2 × 10−37 when compared to Arabidopsis thaliana cryptochrome-1. Residues conserved between the three cryptochromes are marked with the ∧ character.
FIGURE 4
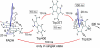
Schematic illustration of electron hole transfer and electron spin dynamics in the FADH cofactor and tryptophan chain. After photoexcitation of the FADH cofactor, an electron hole propagates outward through the three-tryptophan chain (transfer time, 10 ns), forming in sequence the radical-pair states FADH + Trp-400+ → FADH + Trp-377+ → FADH + Trp-324+. The latter radical-pair state is terminated through either electron back-transfer or deprotonation with transition times 100 ns and 300 ns, respectively. The system spends ∼100 ns in the FADH + Trp-324+ state but only 10 ns in the FADH + Trp-400+ or FADH + Trp-377+ states (radical-pair state lifetimes are shown in square boxes), making the FADH + Trp-324+ radical-pair state the major contributor to the magnetic field effect. Electron hole migration (10 ns), spin precession (20 ns), electron back-transfer (100 ns), and deprotonation of Trp-324 (300 ns) are shown with arrows. Also shown are the electronic and nuclear spins in the FADH + Trp-324+ radical pair; in Trp-400 and Trp-377, only the nuclear spins are shown. The nuclear spins are shown with typical random orientations; the electron spins are shown in the initial antiparallel, i.e., singlet, alignment. The picture corresponds to the so-called semi-classical description of electron-nuclear spin dynamics (32,20). In this description, the electron spins ( and
) precess about a local magnetic field produced by the addition of the external magnetic field
and contributions
and
from the nuclear spins on the two radicals. The spin precession continuously alters the relative spin orientation, causing the singlet (antiparallel) ↔ triplet (parallel) interconversion underlying the magnetic field effect. The nuclei which are actually included in our calculations (radical-pair model 2, see text) are labeled.
FIGURE 5
FADH and tryptophan shown with those of their nuclei involved in the strongest hyperfine coupling. The numbering of the nuclei in each radical is chosen to be consistent with that of other studies (64,65,67).
FIGURE 6
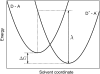
Schematic representation of the energetics assumed in electron transfer theory from a donor D to an acceptor A. The energy, λ, required to reorganize nuclear coordinates upon electron transfer, and the driving force, Δ_G_, for the electron transfer are indicated. The solvent coordinate describes schematically the effect of the protein degrees of freedom on the energy needed to transfer the electron in the process D − A → D+ − _A_−.
FIGURE 7
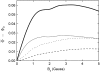
Cryptochrome activation yield for radical-pair model 1. The probability for the formation of FADH + Trp-324dep, averaged over angles θ and φ, for radical-pair model 1, which contains nuclear spins N5 on FADH and H5 and
on the tryptophans, was calculated for different electron back-transfer rate constants: thin solid line, _k_b = 106 _s_−1; thick solid line, _k_b = 107 _s_−1; dotted line, _k_b = 5 × 107 _s_−1; dashed line, _k_b = 108 _s_−1. Φ0 represents the value of the yield at _B_0 = 0. The values of the activation yield at _B_0 = 0 are given in Table 4. The difference in yield over the range from 0 to 5 G is approximately +10% for this model.
FIGURE 8
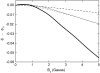
Cryptochrome activation yield for radical-pair model 2. The probability for the formation of FADH + Trp-324dep, averaged over angles θ and φ, for radical-pair model 2, which contains nuclear spins N5 and H5 on FADH and H5 and
on the tryptophans, was calculated for different electron back-transfer rate constants: thin solid line, _k_b = 106 _s_−1; thick solid line, _k_b = 107 _s_−1; dotted line, _k_b = 5 × 107 _s_−1; dashed line, _k_b = 108 _s_−1. Φ0 represents the value of the yield at _B_0 = 0. The values of the activation yield at _B_0 = 0 are given in Table 4. The difference in yield over the range from 0 to 5 G is approximately −11% for this model.
FIGURE 9
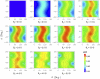
Contour plots of the angular dependence of the cryptochrome activation yield. The plots show the FADH + Trp-324dep yield for radical-pair model 1. The yield exhibits a maximal ridge at about θ = 90°, which is most prominent around _B_0 = 2.5 G and fades away at higher and lower magnetic field strengths.
FIGURE 10
Time dependence of singlet and triplet populations. The results shown are those for radical-pair model 1, calculated for rate constants _k_et = 1 × 108 _s_−1, _k_b = 1 × 107 _s_−1, and evaluated at θ = 0°, φ = 0°.
Similar articles
- Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana.
Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W. Ahmad M, et al. Planta. 2007 Feb;225(3):615-24. doi: 10.1007/s00425-006-0383-0. Epub 2006 Sep 6. Planta. 2007. PMID: 16955271 - Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function.
Zeugner A, Byrdin M, Bouly JP, Bakrim N, Giovani B, Brettel K, Ahmad M. Zeugner A, et al. J Biol Chem. 2005 May 20;280(20):19437-40. doi: 10.1074/jbc.C500077200. Epub 2005 Mar 17. J Biol Chem. 2005. PMID: 15774475 - Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark.
Pooam M, Arthaut LD, Burdick D, Link J, Martino CF, Ahmad M. Pooam M, et al. Planta. 2019 Feb;249(2):319-332. doi: 10.1007/s00425-018-3002-y. Epub 2018 Sep 7. Planta. 2019. PMID: 30194534 - A radical sense of direction: signalling and mechanism in cryptochrome magnetoreception.
Dodson CA, Hore PJ, Wallace MI. Dodson CA, et al. Trends Biochem Sci. 2013 Sep;38(9):435-46. doi: 10.1016/j.tibs.2013.07.002. Epub 2013 Aug 9. Trends Biochem Sci. 2013. PMID: 23938034 Review. - Tales from the crypt(ochromes).
Van Gelder RN. Van Gelder RN. J Biol Rhythms. 2002 Apr;17(2):110-20. doi: 10.1177/074873002129002401. J Biol Rhythms. 2002. PMID: 12002158 Review.
Cited by
- Compass magnetoreception in birds arising from photo-induced radical pairs in rotationally disordered cryptochromes.
Lau JC, Rodgers CT, Hore PJ. Lau JC, et al. J R Soc Interface. 2012 Dec 7;9(77):3329-37. doi: 10.1098/rsif.2012.0374. Epub 2012 Sep 12. J R Soc Interface. 2012. PMID: 22977104 Free PMC article. - Vibrationally assisted electron transfer mechanism of olfaction: myth or reality?
Solov'yov IA, Chang PY, Schulten K. Solov'yov IA, et al. Phys Chem Chem Phys. 2012 Oct 28;14(40):13861-71. doi: 10.1039/c2cp41436h. Epub 2012 Aug 17. Phys Chem Chem Phys. 2012. PMID: 22899100 Free PMC article. - Rotations of macromolecules affect nonspecific biological responses to magnetic fields.
Binhi VN, Prato FS. Binhi VN, et al. Sci Rep. 2018 Sep 10;8(1):13495. doi: 10.1038/s41598-018-31847-y. Sci Rep. 2018. PMID: 30202025 Free PMC article. - Cryptochromes--a potential magnetoreceptor: what do we know and what do we want to know?
Liedvogel M, Mouritsen H. Liedvogel M, et al. J R Soc Interface. 2010 Apr 6;7 Suppl 2(Suppl 2):S147-62. doi: 10.1098/rsif.2009.0411.focus. Epub 2009 Nov 11. J R Soc Interface. 2010. PMID: 19906675 Free PMC article. - Influence of Geomagnetic Disturbances at Different Times of Day on Locomotor Activity in Zebrafish (Danio Rerio).
Krylov VV. Krylov VV. Clocks Sleep. 2021 Nov 29;3(4):624-632. doi: 10.3390/clockssleep3040045. Clocks Sleep. 2021. PMID: 34940024 Free PMC article.
References
- von Middendorff, A. 1859. Die Isepiptesen Rußlands. Mem. Acad. Sci. St. Petersbourg VI Ser. 8:1–43.
- Viguier, C. 1882. Le sens de l'orientation et ses organes ches les animaux et chez l'homme. Revue Philosophique de la France et de l'Etranger. 14:1–36.
- Wiltschko, W., and F. Merkel. 1966. Orientierung zugunruhiger Rotkehlchen im statischen Magnetfeld. Verh. dt. Zool. Ges. 59:362–367.
- Wiltschko, W., and R. Wiltschko. 2002. Magnetic compass orientation in birds and its physiological basis. Naturwissenschaften. 89:445–452. - PubMed
- Wiltschko, W., and R. Wiltschko. 2005. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. [A]. 191:675–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical