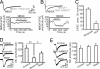mGluR5 stimulates gliotransmission in the nucleus accumbens - PubMed (original) (raw)
mGluR5 stimulates gliotransmission in the nucleus accumbens
Marcello D'Ascenzo et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Although metabotropic glutamate receptor 5 (mGluR5) is essential for cocaine self-administration and drug-seeking behavior, there is limited knowledge of the cellular actions of this receptor in the nucleus accumbens (NAc). Although mGluR5 has the potential to regulate neurons directly, recent studies have shown the importance of mGluR5 in regulating Ca(2+) signaling in astrocytes and, as a consequence, the Ca(2+)-dependent release of excitatory transmitters from these glia. In this study, we demonstrate that activation of mGluR5 induces Ca(2+) oscillations in NAc astrocytes with the correlated appearance of NMDA receptor-dependent slow inward currents detected in medium spiny neurons (MSNs). Photolysis of caged Ca(2+) loaded specifically into astrocytes evoked slow inward currents demonstrating that Ca(2+) elevations in astrocytes are responsible for these excitatory events. Pharmacological evaluation of these glial-evoked NMDA currents shows that they are mediated by NR2B-containing NMDA receptors, whereas synaptic NMDA receptors rely on NR2A-containing receptors. Stimulation of glutamatergic afferents activates mGluR5-dependent astrocytic Ca(2+) oscillations and gliotransmission that is sustained for minutes beyond the initial stimulus. Because gliotransmission is mediated by NMDA receptors, depolarized membrane potentials exhibited during up-states augment excitation provided by gliotransmission, which drives bursts of MSN action potentials. Because the predominant mGluR5-dependent action of glutamatergic afferents is to cause the sustained activation of astrocytes, which in turn excite MSNs through extrasynaptic NMDA receptors, our results raise the potential for gliotransmission being involved in prolonged mGluR5-dependent adaptation in the NAc.
Conflict of interest statement
Conflict of interest statement: P.G.H. has equity interest in the company Prairie Technologies, Inc., which manufactures the two-photon microscope used in this study.
Figures
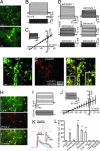
Fig. 1.
NAc astrocytes are electrically inexcitable, are electrotonically coupled with one another, express mGluR5 receptors, and respond to neurotransmitters with Ca2+ oscillations. (A) Two-photon laser-scanning image of GFP-expressing cells from a NAc slice obtained from pGFAP-GFP transgenic mice (_z_-projection of 15 planes). (Scale bar: Upper, 40 μm; Lower, 10 μm.) An astrocytic endfoot making contact with a capillary (∗). (B) Typical response of a NAc pGFAP-GFP+ cell to current injections from −0.2 to 1.1 nA (bottom traces, 0.1-nA increments). (C) Average whole-cell I–V relationship from GFP-expressing cells (n = 15 cells). (Inset) Inward and outward currents recorded in voltage-clamp configurations (−135 to +25 mV, 10-mV increments). (Scale bars: 2 nA, 50 ms.) (D) Paired recordings from two GFP-positive cells showing bidirectional coupling. (E–G) mGluR5 is expressed in NAc astrocytes. Top-view reconstruction obtained from confocal _z_-series of GFP-expressing astrocytes (E, green). mGluR5 immunoreactivity (F, red) associated with GFP-positive astrocytic processes reveals localization of this receptor mainly in astrocytic processes rather than in astrocytic cell bodies (G). [Note that we used an image mask based on the GFP fluorescence of the astrocyte to display pixels displaying mGluR5 immunoreactivity associated with astrocytes (see
SI Materials and Methods
).] Insets in G show regions 1 and 2 at higher magnification. (Scale bars: E–G, 20 μm; G Inset, 10 μm.) (F Inset) Western blot analysis from isolated NAc slices confirm the presence of the mGluR5 in this brain region. (H) NAc slices from pGFAP-GFP mice loaded with Rhod-2 (red) showing selective labeling of GFP expressing astrocytes (green). (Scale bar: 20 μm.) (I) Current-clamp recordings from a dye-loaded cell showing astrocytic membrane properties and no action potential firing. (J) Average whole-cell I–V relationship from dye-loaded cells (n = 3 cells) and representative currents after voltage steps from −115 mV to +25 mV (Inset). (K) Astrocyte responses (time course of the Δ_F_/F ratio) to DHPG (20 μM; Upper) and ATP (100 μM; Lower). (L) Average percentage of astrocytes displaying Ca2+ oscillations under the different experimental conditions: ACSF, n = 23 slices; DHPG (20 μM), n = 4 slices; DHPG (20 μM) + MPEP (50 μM), n = 4 slices; ATP (50/100 μM), n = 5 slices; baclofen (40 μM), n = 4 slices; low Ca2+, n = 5 slices; SKF 38393 (2.5–5 μM), n = 3 slices; quinpirole (20 μM), n = 3 slices; and cocaine (1–10 μM), n = 4 slices.
Fig. 2.
SICs evoked in MSNs by stimuli that induce astrocytic Ca2+ oscillations. (A) Patch-clamp recording from an MSN in voltage-clamp showing a spontaneous SIC after slice perfusion with 0 extracellular Mg2+ ACSF. Note the slow kinetics typical of SICs compared with miniature postsynaptic currents. _V_h = −85 mV. (B) Histogram of rise (Left; 259 events; bin, 5 ms) and decay (Center; 259 events; bin, 50 ms) time of SICs. (Right) Overlay of an SIC (black trace) and an EPSC (red trace). (C) Representative paired-recording from two MSNs showing synchronous SICs. (D) Interevent time interval histogram of SICs occurring in pairs of recorded MSNs (n = 7 pairs). Of a total of 55 SICs, 16 SICs were recorded within a time window of 200 ms. Bins, 50 ms. (E) SICs induced by perfusion with DHPG (20 μM); ∗ indicates SIC. (F) Average SIC frequency in ACSF, n = 106 cells; 0 mM Mg2+, n = 147 cells; DHPG (20 μM), n = 15 cells; DHPG (20 μM) + MPEP (50 μM), n = 5 cells; ATP (50 μM), n = 9 cells; baclofen (40 μM), n = 6 cells; SKF 38393 (2.5–5 μM), n = 5 cells; quinpirole (20 μM), n = 5 cells; cocaine (1–10 μM), n = 9 cells; and low Ca2+, n = 95 cells.
Fig. 3.
NR2B-containing NMDA receptors are the preferential target of astrocytic glutamate. (A) Ifenprodil (5/10 μM) does not reduce the NMDA EPSC amplitude [averages of 10 excitatory postsynaptic potentials (EPSPs) in each condition]. Experiments performed in the presence of NBQX (30 μM) and 0 Mg2+. (B) NR2A antagonist NVP-AAM077 (0.4 μM) reduces NMDA receptor-mediated EPSC amplitude. (C) Average amplitude of the NMDA EPSCs in the presence of ifenprodil (n = 6 cells) and NVP-AAM077 (n = 6 cells). Data are normalized to NMDA EPSC amplitude before antagonist application. (D) Ifenprodil (5–10 μM) reduces SIC amplitude. (E) NR2A antagonist NVP-AAM077 (0.4 μM) does not affect SIC amplitude.
Fig. 4.
Glutamatergic afferents activate mGluR5-dependent gliotransmission. (A) mGluR5 receptors do not modulate glutamatergic synaptic transmission. (A Left) Field EPSPs under control conditions, in the presence of DHPG (20 μM) and NBQX (30 μM). The arrow indicates the EPSP. (A Right) Average slope of field EPSPs in the presence of MPEP (50 μM) and DHPG (20 μM) (n = 6 slices). (B) Whole-cell recording showing that EPSC amplitude is not affected by DHPG application (n = 6 cells). (C) Astrocyte responses (time course of the Δ_F_/F ratio) to glutamatergic afferent stimulation. Afferents were stimulated by using 10 trains of seven stimuli delivered at 30 Hz. (D) Average percentage of astrocytes displaying Ca2+ oscillations during the sequential experimental conditions (n = 5): glutamatergic afferent stimulation, in ACSF, MPEP (50 μM), after washout of MPEP, and in the presence of TTX (1 μM). (E) Whole-cell recordings in Mg2+-free ACSF showing two SICs triggered by stimulus trains (10 trains of seven stimuli delivered at 30 Hz) applied to the glutamatergic afferents. See Inset for one train of seven stimuli at an expanded time scale. (Scale bar: 50 ms, 50 pA.) Note that stimulation of glutamatergic afferent elicits a prolonged increase in SIC frequency. (F) Average SIC frequency in a 3-min time period before and after glutamatergic afferent stimulation (n = 9 cells). (G) Blockade of mGluR5 by MPEP prevents the ability of glutamatergic afferent stimulation to trigger SICs (MPEP 50 μM, n = 5).
Fig. 5.
Gliotransmission drives increased action potential generation in MSNs at up-state membrane potentials. (A) Average traces of SICs (n = 19) at holding potentials of −85 and −65 mV showing the enhancement of SIC amplitude at up-state (−65 mV) compared with down-state (−85 mV) membrane potentials (error bars are SEM). All experiments reported in this figure were made in 1 mM Mg2+-containing ACSF. (B) Average amplitude of the SICs at the two different holding potentials. (C) Average SIC frequency after stimulation of class I mGluRs with DHPG (20 μM) at _V_h of −85 and −65 mV (n = 10). (D) The EPSC (Left) and pure NMDA-mediated EPSC (Right) at holding potentials of −85 and −65 mV. NMDA currents were recorded in NBQX (30 μM). Note the reduction of EPSC amplitude at up-state (−65 mV) compared with down-state (−85 mV) membrane potentials in contrast to the augmentation of the SIC (A). Traces in D are averages of 10 evoked EPSCs. (E) −65/−85 mV ratio of amplitude the EPSC and pure NMDA-mediated EPSC currents at the two different voltages (n = 5). (F) Paired whole-cell recordings from two closely spaced MSNs in Mg2+-containing ACSF. Glutamatergic afferents were stimulated with 10 trains of seven 30-Hz stimuli (one train shown) that evoked EPSPs in the neuron recorded in current-clamp (Upper) and EPSCs in the voltage-clamped neuron (Lower). With a delay after afferent stimulation, synchronous excitation of paired MSNs show that gliotransmission powerfully excites MSNs to generate a burst of action potentials. (G) In contrast, gliotransmission evokes few action potentials when MSNs are at down-state membrane potentials. (H) Comparison on the kinetics of SICs at up- and down-state membrane potentials (n = 13 events). (I) Average number of action potentials during gliotransmission-mediated depolarization (black bars, n = 6; gray bars, n = 5).
Similar articles
- Activation of mGluR5 induces spike afterdepolarization and enhanced excitability in medium spiny neurons of the nucleus accumbens by modulating persistent Na+ currents.
D'Ascenzo M, Podda MV, Fellin T, Azzena GB, Haydon P, Grassi C. D'Ascenzo M, et al. J Physiol. 2009 Jul 1;587(Pt 13):3233-50. doi: 10.1113/jphysiol.2009.172593. Epub 2009 May 11. J Physiol. 2009. PMID: 19433572 Free PMC article. - Insights into the release mechanism of astrocytic glutamate evoking in neurons NMDA receptor-mediated slow depolarizing inward currents.
Gómez-Gonzalo M, Zehnder T, Requie LM, Bezzi P, Carmignoto G. Gómez-Gonzalo M, et al. Glia. 2018 Oct;66(10):2188-2199. doi: 10.1002/glia.23473. Epub 2018 Aug 25. Glia. 2018. PMID: 30144319 - Astrocytes control neuronal excitability in the nucleus accumbens.
Fellin T, D'Ascenzo M, Haydon PG. Fellin T, et al. ScientificWorldJournal. 2007 Nov 2;7:89-97. doi: 10.1100/tsw.2007.195. ScientificWorldJournal. 2007. PMID: 17982581 Free PMC article. Review. - Gliotransmission: Beyond Black-and-White.
Savtchouk I, Volterra A. Savtchouk I, et al. J Neurosci. 2018 Jan 3;38(1):14-25. doi: 10.1523/JNEUROSCI.0017-17.2017. J Neurosci. 2018. PMID: 29298905 Free PMC article. Review.
Cited by
- Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine.
Baker DA, Madayag A, Kristiansen LV, Meador-Woodruff JH, Haroutunian V, Raju I. Baker DA, et al. Neuropsychopharmacology. 2008 Jun;33(7):1760-72. doi: 10.1038/sj.npp.1301532. Epub 2007 Aug 29. Neuropsychopharmacology. 2008. PMID: 17728701 Free PMC article. - Sensory and cortical activation of distinct glial cell subtypes in the somatosensory thalamus of young rats.
Parri HR, Gould TM, Crunelli V. Parri HR, et al. Eur J Neurosci. 2010 Jul;32(1):29-40. doi: 10.1111/j.1460-9568.2010.07281.x. Eur J Neurosci. 2010. PMID: 20608967 Free PMC article. - Astrocyte Gliotransmission in the Regulation of Systemic Metabolism.
Murat CB, García-Cáceres C. Murat CB, et al. Metabolites. 2021 Oct 26;11(11):732. doi: 10.3390/metabo11110732. Metabolites. 2021. PMID: 34822390 Free PMC article. Review. - Metabotropic glutamate receptors in glial cells.
D'Antoni S, Berretta A, Bonaccorso CM, Bruno V, Aronica E, Nicoletti F, Catania MV. D'Antoni S, et al. Neurochem Res. 2008 Dec;33(12):2436-43. doi: 10.1007/s11064-008-9694-9. Epub 2008 Apr 26. Neurochem Res. 2008. PMID: 18438710 Review. - Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse?
Haydon PG, Blendy J, Moss SJ, Rob Jackson F. Haydon PG, et al. Neuropharmacology. 2009;56 Suppl 1(Suppl 1):83-90. doi: 10.1016/j.neuropharm.2008.06.050. Epub 2008 Jul 3. Neuropharmacology. 2009. PMID: 18647612 Free PMC article. Review.
References
- Kalivas PW. Curr Opin Pharmacol. 2004;4:23–29. - PubMed
- Wolf ME. Prog Neurobiol. 1998;54:679–720. - PubMed
- Vanderschuren LJ, Kalivas PW. Psychopharmacology (Berlin) 2000;151:99–120. - PubMed
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Nat Neurosci. 2001;4:873–874. - PubMed
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Eur J Pharmacol. 2004;499:121–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS043142/NS/NINDS NIH HHS/United States
- R01 NS048045/NS/NINDS NIH HHS/United States
- R01NS043142/NS/NINDS NIH HHS/United States
- NS048045/NS/NINDS NIH HHS/United States
- NS051195/NS/NINDS NIH HHS/United States
- R01 NS046478/NS/NINDS NIH HHS/United States
- R01 NS051195/NS/NINDS NIH HHS/United States
- P20MH071705/MH/NIMH NIH HHS/United States
- NS046478/NS/NINDS NIH HHS/United States
- P30 NS047321/NS/NINDS NIH HHS/United States
- R37 NS037585/NS/NINDS NIH HHS/United States
- P20 MH071705/MH/NIMH NIH HHS/United States
- P30NS047321/NS/NINDS NIH HHS/United States
- R37NS037585/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous