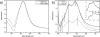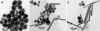Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli - PubMed (original) (raw)
Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli
Sukdeb Pal et al. Appl Environ Microbiol. 2007 Mar.
Abstract
In this work we investigated the antibacterial properties of differently shaped silver nanoparticles against the gram-negative bacterium Escherichia coli, both in liquid systems and on agar plates. Energy-filtering transmission electron microscopy images revealed considerable changes in the cell membranes upon treatment, resulting in cell death. Truncated triangular silver nanoplates with a {111} lattice plane as the basal plane displayed the strongest biocidal action, compared with spherical and rod-shaped nanoparticles and with Ag(+) (in the form of AgNO(3)). It is proposed that nanoscale size and the presence of a {111} plane combine to promote this biocidal property. To our knowledge, this is the first comparative study on the bactericidal properties of silver nanoparticles of different shapes, and our results demonstrate that silver nanoparticles undergo a shape-dependent interaction with the gram-negative organism E. coli.
Figures
FIG. 1.
(a) Absorption spectra of solutions containing spherical silver nanoparticle recorded immediately after precipitation and after 1 week. (b) Absorption spectra of reaction mixtures containing elongated (rod-shaped) and truncated triangular silver nanoplates and other anisotropic silver nanoparticles and the first and second wash liquor (centrifuge). (Top inset) Absorption spectrum of solution containing purified truncated triangular silver nanoplates. (Bottom inset) UV-visible spectrum of the solution containing rod-shaped particles with larger aspect ratios.
FIG. 2.
EFTEM images of silver nanoparticles. (A) Spherical nanoparticles synthesized by citrate reduction. (B) Silver nanoparticles of different shapes. (C) Purified rod-shaped nanoparticles.
FIG. 3.
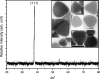
OPML-XRD pattern of truncated triangular silver nanoplates. (Inset) TEM image of the purified truncated triangular particles.
FIG. 4.
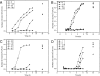
(A) Petri dishes initially supplemented with 107 CFU/ml of E. coli and incubated with different forms of silver nanoparticles at (a) 1, (b) 12.5, (c) 50, and (d) 100 μg. (B) Number of E. coli colonies, expressed as log(1 + number of colonies grown on plates under the conditions used for panel A), as a function of the amount of silver nanoparticles in agar plates.
FIG. 5.
Petri dishes initially supplemented with 105 CFU/ml of E. coli and incubated with silver at (a) 1, (b) 6, (c) 12.5, (d) 50, and (e) 100 μg and corresponding graphs showing log(1 + number of colonies grown on plates) as a function of the concentration of silver in agar plates. (A) Ag+ (in the form of AgNO3); (B) spherical silver nanoparticles.
FIG. 6.
Growth curve of E. coli in 100 ml NB with 107 CFU/ml in the presence of different concentrations of different silver nanoparticles. (A) AgNO3; (B) spherical nanoparticles; (C) truncated triangular nanoparticles; (D) spherical nanoparticles with an initial bacterial concentration of 105 CFU/ml. Amounts (in micrograms) of silver nanoparticles are given in each panel.
FIG. 7.
(a) Killing activity for E. coli (in 100 ml NB supplemented with 107 CFU/ml) in the presence of a mixture of triangular, rod-shaped, and polyhedral silver nanoparticles (before purification), containing different amounts of total silver (Ag+ and Ag nanoparticles) and CTAB. Total silver amounts are shown in each panel. (b) Decay curve of E. coli growth in 100 ml NB with 107 CFU/ml treated with the mother solution of truncated triangular and rod-shaped nanoparticles (total amount of silver, 12 μg). (Inset) Comparative graph of the dynamics of E. coli growth in the presence of 12 μg of spherical silver nanoparticles, 10 μg of triangular nanoparticles, and 12 μg of ionic silver (AgNO3) in 100 ml NB supplemented with 107 CFU/ml. Axes and units for the inset are same as those for the main graph and have been omitted for simplicity.
FIG. 8.
EFTEM images of E. coli cells. (A) Untreated E. coli. Flagella can be seen. (B) E. coli grown on agar plates supplemented with Ag+ (AgNO3). Arrows indicate partially damaged membranes. These cells are viable. (C) E. coli treated with triangular silver nanoplates. Silver nanoparticles appear as dark irregular pits on the cell surface. (D) E. coli treated with spherical silver nanoparticles. (E) Enlarged image of part of the bacterial cell membrane treated with triangular silver nanoparticles. The cell membrane is damaged in multiple locations.
Similar articles
- Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria.
Sondi I, Salopek-Sondi B. Sondi I, et al. J Colloid Interface Sci. 2004 Jul 1;275(1):177-82. doi: 10.1016/j.jcis.2004.02.012. J Colloid Interface Sci. 2004. PMID: 15158396 - Silver nanoparticle-E. coli colloidal interaction in water and effect on E. coli survival.
Dror-Ehre A, Mamane H, Belenkova T, Markovich G, Adin A. Dror-Ehre A, et al. J Colloid Interface Sci. 2009 Nov 15;339(2):521-6. doi: 10.1016/j.jcis.2009.07.052. Epub 2009 Jul 28. J Colloid Interface Sci. 2009. PMID: 19726047 - Ag colloids and Ag clusters over EDAPTMS-coated silica nanoparticles: synthesis, characterization, and antibacterial activity against Escherichia coli.
Rastogi SK, Rutledge VJ, Gibson C, Newcombe DA, Branen JR, Branen AL. Rastogi SK, et al. Nanomedicine. 2011 Jun;7(3):305-14. doi: 10.1016/j.nano.2010.11.003. Epub 2010 Nov 19. Nanomedicine. 2011. PMID: 21094275 Free PMC article. - Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways.
Song Z, Wu Y, Wang H, Han H. Song Z, et al. Mater Sci Eng C Mater Biol Appl. 2019 Jun;99:255-263. doi: 10.1016/j.msec.2018.12.053. Epub 2019 Jan 19. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30889699 - Modification of Silver Nanoplates with Cell-Binding Subunit of Bacterial Toxin and Their Antimicrobial Activity against Intracellular Bacteria.
Harada A, Xu W, Ono K, Tsutsuki H, Yahiro K, Sawa T, Niidome T. Harada A, et al. ACS Appl Bio Mater. 2023 Sep 18;6(9):3387-3394. doi: 10.1021/acsabm.3c00019. Epub 2023 Mar 27. ACS Appl Bio Mater. 2023. PMID: 36972339 Review.
Cited by
- Organic-inorganic hybrid nanoparticles for bacterial inhibition: synthesis and characterization of doped and undoped ONPs with Ag/Au NPs.
Aguilar CA, Jiménez AB, Silva AR, Kaur N, Thangarasu P, Ramos JM, Singh N. Aguilar CA, et al. Molecules. 2015 Apr 7;20(4):6002-21. doi: 10.3390/molecules20046002. Molecules. 2015. PMID: 25853317 Free PMC article. - Thermal co-reduction approach to vary size of silver nanoparticle: its microbial and cellular toxicology.
Dasgupta N, Ranjan S, Rajendran B, Manickam V, Ramalingam C, Avadhani GS, Kumar A. Dasgupta N, et al. Environ Sci Pollut Res Int. 2016 Mar;23(5):4149-63. doi: 10.1007/s11356-015-4570-z. Epub 2015 May 6. Environ Sci Pollut Res Int. 2016. PMID: 25943508 - Nanolayered Metal Phosphates as Biocompatible Reservoirs for Antimicrobial Silver Nanoparticles.
García I, Trobajo C, Amghouz Z, Adawy A. García I, et al. Materials (Basel). 2021 Mar 18;14(6):1481. doi: 10.3390/ma14061481. Materials (Basel). 2021. PMID: 33803515 Free PMC article. - Comparative antibacterial activity of silver nanoparticles synthesised by biological and chemical routes with pluronic F68 as a stabilising agent.
Santos CA, Seckler MM, Ingle AP, Rai M. Santos CA, et al. IET Nanobiotechnol. 2016 Aug;10(4):200-5. doi: 10.1049/iet-nbt.2015.0055. IET Nanobiotechnol. 2016. PMID: 27463790 Free PMC article. - Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties.
Duman H, Eker F, Akdaşçi E, Witkowska AM, Bechelany M, Karav S. Duman H, et al. Nanomaterials (Basel). 2024 Sep 20;14(18):1527. doi: 10.3390/nano14181527. Nanomaterials (Basel). 2024. PMID: 39330683 Free PMC article. Review.
References
- Ajayan, P. M., and L. D. Marks. 1988. Quasimelting and phases of small particles. Phys. Rev. Lett. 60:585-587. - PubMed
- Bosetti, M., A. Masse, E. Tobin, and M. Cannas. 2002. Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomaterials 23:887-892. - PubMed
- Bragg, P. D., and D. J. Rainnie. 1974. The effect of silver ions on the respiratory chains of Escherichia coli. Can. J. Microbiol. 20:883-889. - PubMed
- Brause, R., H. Moeltgen, and K. Kleinermanns. 2002. Characterization of laser-ablated and chemically reduced silver colloids in aqueous solution by UV/VIS spectroscopy and STM/SEM microscopy. Appl. Phys. B 75:711-716.
- Brigger, I., C. Dubernet, and P. Couvreur. 2002. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Delivery Rev. 54:631-651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical