Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA-induced rat mammary tumors - PubMed (original) (raw)
Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA-induced rat mammary tumors
Anette Raa et al. BMC Cancer. 2007.
Abstract
Background: This study investigated the effects of hyperoxic treatment on growth, angiogenesis, apoptosis, general morphology and gene expression in DMBA-induced rat mammary tumors.
Methods: One group of animals was exposed to normobaric hyperoxia (1 bar, pO2 = 1.0 bar) and another group was exposed to hyperbaric hyperoxia (1.5 bar, pO2 = 1.5 bar). A third group was treated with the commonly used chemotherapeutic drug 5- Fluorouracil (5-FU), whereas animals housed under normal atmosphere (1 bar, pO2 = 0.2 bar) served as controls. All treatments were performed on day 1, 4, 7 and 10 for 90 min. Tumor growth was calculated from caliper measurements. Biological effects of the treatment, was determined by assessment of vascular morphology (immunostaining for von Willebrandt factor) and apoptosis (TUNEL staining). Detailed gene expression profiles were obtained and verified by quantitative rtPCR.
Results: Tumor growth was significantly reduced (~57-66 %) after hyperoxic treatment compared to control and even more than 5-FU (~36 %). Light microscopic observations of the tumor tissue showed large empty spaces within the tissue after hyperoxic treatment, probably due to loss of glands as indicated by a strong down-regulation of glandular secretory proteins. A significant reduction in mean vascular density (30-50%) was found after hyperoxic treatment. Furthermore, increased apoptosis (18-21%) was found after hyperoxic treatment.
Conclusion: Thus, by increasing the pO2 in mammary tumor tissue using normobaric and moderate hyperbaric oxygen therapy, a significant retardation in tumor growth is achieved, by loss of glands, reduction in vascular density and enhanced cell death. Hyperbaric oxygen should therefore be further evaluated as a tumor treatment.
Figures
Figure 1
The effect of normoxic and hyperoxic treatment on tumor growth compared to controls and 5-FU treated. Treatments were given on day 1, 4, 7 and 10. Values represent means ± SE.
Figure 2
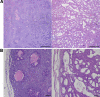
Examples of eosin-hematoxylin stained tumor-tissue of the central (A) and peripheral (B) part of the mammary tumor in control (left) and during hyperoxic treatment (right, 1 bar, pO2 = 1.0). The images under A are scaled to the same magnification (× 4) and the images under B to the same magnification (× 10). Scale bar indicate 500 μm (A) and 100 μm (B).
Figure 3
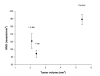
The relationship between tumor size and blood vessels per mm2 before and after hyperoxic treatment. Values represents mean ± SE.
Similar articles
- Hyperoxia retards growth and induces apoptosis, changes in vascular density and gene expression in transplanted gliomas in nude rats.
Stuhr LE, Raa A, Oyan AM, Kalland KH, Sakariassen PO, Petersen K, Bjerkvig R, Reed RK. Stuhr LE, et al. J Neurooncol. 2007 Nov;85(2):191-202. doi: 10.1007/s11060-007-9407-2. Epub 2007 Jun 8. J Neurooncol. 2007. PMID: 17557137 - Hyperbaric oxygen alone or combined with 5-FU attenuates growth of DMBA-induced rat mammary tumors.
Stuhr LE, Iversen VV, Straume O, Maehle BO, Reed RK. Stuhr LE, et al. Cancer Lett. 2004 Jul 8;210(1):35-40. doi: 10.1016/j.canlet.2004.02.012. Cancer Lett. 2004. PMID: 15172118 - Natural and synthetic progestins accelerate 7,12-dimethylbenz[a]anthracene-initiated mammary tumors and increase angiogenesis in Sprague-Dawley rats.
Benakanakere I, Besch-Williford C, Schnell J, Brandt S, Ellersieck MR, Molinolo A, Hyder SM. Benakanakere I, et al. Clin Cancer Res. 2006 Jul 1;12(13):4062-71. doi: 10.1158/1078-0432.CCR-06-0427. Clin Cancer Res. 2006. PMID: 16818706 - High-dose, short-term, anti-inflammatory treatment with dexamethasone reduces growth and augments the effects of 5-fluorouracil on dimethyl-alpha-benzanthracene-induced mammary tumors in rats.
Stuhr LE, Salnikov AV, Iversen VV, Salvesen G, Rubin K, Reed RK. Stuhr LE, et al. Scand J Clin Lab Invest. 2006;66(6):477-86. doi: 10.1080/00365510600788332. Scand J Clin Lab Invest. 2006. PMID: 17000555 - Effects of hyperbaric and hyperoxic conditions on the disposition of drugs: theoretical considerations and a review of the literature.
Rump AF, Siekmann U, Kalff G. Rump AF, et al. Gen Pharmacol. 1999 Jan;32(1):127-33. doi: 10.1016/s0306-3623(98)00074-3. Gen Pharmacol. 1999. PMID: 9888265 Review.
Cited by
- Can we negotiate with a tumor?
Wolfrom CM, Laurent M, Deschatrette J. Wolfrom CM, et al. PLoS One. 2014 Aug 1;9(8):e103834. doi: 10.1371/journal.pone.0103834. eCollection 2014. PLoS One. 2014. PMID: 25084359 Free PMC article. - Ambient oxygen promotes tumorigenesis.
Sung HJ, Ma W, Starost MF, Lago CU, Lim PK, Sack MN, Kang JG, Wang PY, Hwang PM. Sung HJ, et al. PLoS One. 2011 May 12;6(5):e19785. doi: 10.1371/journal.pone.0019785. PLoS One. 2011. PMID: 21589870 Free PMC article. - Kallistatin suppresses cancer development by multi-factorial actions.
Chao J, Li P, Chao L. Chao J, et al. Crit Rev Oncol Hematol. 2017 May;113:71-78. doi: 10.1016/j.critrevonc.2017.03.011. Epub 2017 Mar 14. Crit Rev Oncol Hematol. 2017. PMID: 28427524 Free PMC article. Review. - Metabolic pathway for the universal fluorescent recognition of tumor cells.
Fernandez-Carrascal A, Garcia-Algar M, Nazarenus M, Torres-Nuñez A, Guerrini L, Feliu N, Parak WJ, Garcia-Rico E, Alvarez-Puebla RA. Fernandez-Carrascal A, et al. Oncotarget. 2017 Jun 16;8(44):76108-76115. doi: 10.18632/oncotarget.18551. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100296 Free PMC article. - α-Ketoglutarate attenuates Wnt signaling and drives differentiation in colorectal cancer.
Tran TQ, Hanse EA, Habowski AN, Li H, Ishak Gabra MB, Yang Y, Lowman XH, Ooi AM, Liao SY, Edwards RA, Waterman ML, Kong M. Tran TQ, et al. Nat Cancer. 2020 Mar;1(3):345-358. doi: 10.1038/s43018-020-0035-5. Epub 2020 Mar 20. Nat Cancer. 2020. PMID: 32832918 Free PMC article.
References
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. Review. - PubMed
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–91. Review. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources