Context based mixture model for cell phase identification in automated fluorescence microscopy - PubMed (original) (raw)
Context based mixture model for cell phase identification in automated fluorescence microscopy
Meng Wang et al. BMC Bioinformatics. 2007.
Abstract
Background: Automated identification of cell cycle phases of individual live cells in a large population captured via automated fluorescence microscopy technique is important for cancer drug discovery and cell cycle studies. Time-lapse fluorescence microscopy images provide an important method to study the cell cycle process under different conditions of perturbation. Existing methods are limited in dealing with such time-lapse data sets while manual analysis is not feasible. This paper presents statistical data analysis and statistical pattern recognition to perform this task.
Results: The data is generated from Hela H2B GFP cells imaged during a 2-day period with images acquired 15 minutes apart using an automated time-lapse fluorescence microscopy. The patterns are described with four kinds of features, including twelve general features, Haralick texture features, Zernike moment features, and wavelet features. To generate a new set of features with more discriminate power, the commonly used feature reduction techniques are used, which include Principle Component Analysis (PCA), Linear Discriminant Analysis (LDA), Maximum Margin Criterion (MMC), Stepwise Discriminate Analysis based Feature Selection (SDAFS), and Genetic Algorithm based Feature Selection (GAFS). Then, we propose a Context Based Mixture Model (CBMM) for dealing with the time-series cell sequence information and compare it to other traditional classifiers: Support Vector Machine (SVM), Neural Network (NN), and K-Nearest Neighbor (KNN). Being a standard practice in machine learning, we systematically compare the performance of a number of common feature reduction techniques and classifiers to select an optimal combination of a feature reduction technique and a classifier. A cellular database containing 100 manually labelled subsequence is built for evaluating the performance of the classifiers. The generalization error is estimated using the cross validation technique. The experimental results show that CBMM outperforms all other classifies in identifying prophase and has the best overall performance.
Conclusion: The application of feature reduction techniques can improve the prediction accuracy significantly. CBMM can effectively utilize the contextual information and has the best overall performance when combined with any of the previously mentioned feature reduction techniques.
Figures
Figure 1
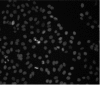
A gray level image of a population of cells showing only nuclei channel. The image shows the nuclei after image enhancements.
Figure 4
Changes in the appearance of a nucleus during cell mitosis. From (a) to (h) consecutive image subframes form a sequence showing nuclei size and shape changes during cell mitosis.
Figure 5
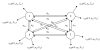
An example of Continuous Gaussian Mixture Hidden Markov Model. M = 4, R = 2, the prior probability of phases are π i, i = 1,2,3,4 which are ignored in this picture.
Figure 2
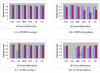
Charts of average precision and sensitivity by CBMM and SVM. Charts (A), (C) show the average precision and sensitivity of different classifiers; while (B), (D) show the precision and sensitivity of the prophase cell identified by different classifiers.
Figure 3
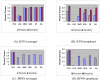
Charts of average precision and sensitivity by KNN and BPNN. Charts (A), (C) show the average precision and sensitivity of different classifiers; while (B), (D) show the precision and sensitivity of the prophase cell identified by different classifiers.
Similar articles
- Novel cell segmentation and online SVM for cell cycle phase identification in automated microscopy.
Wang M, Zhou X, Li F, Huckins J, King RW, Wong ST. Wang M, et al. Bioinformatics. 2008 Jan 1;24(1):94-101. doi: 10.1093/bioinformatics/btm530. Epub 2007 Nov 7. Bioinformatics. 2008. PMID: 17989093 - Computer-assisted lip diagnosis on Traditional Chinese Medicine using multi-class support vector machines.
Li F, Zhao C, Xia Z, Wang Y, Zhou X, Li GZ. Li F, et al. BMC Complement Altern Med. 2012 Aug 16;12:127. doi: 10.1186/1472-6882-12-127. BMC Complement Altern Med. 2012. PMID: 22898352 Free PMC article. - Automated analysis of the mitotic phases of human cells in 3D fluorescence microscopy image sequences.
Harder N, Mora-Bermúdez F, Godinez WJ, Ellenberg J, Eils R, Rohr K. Harder N, et al. Med Image Comput Comput Assist Interv. 2006;9(Pt 1):840-8. doi: 10.1007/11866565_103. Med Image Comput Comput Assist Interv. 2006. PMID: 17354969 - Building cell models and simulations from microscope images.
Murphy RF. Murphy RF. Methods. 2016 Mar 1;96:33-39. doi: 10.1016/j.ymeth.2015.10.011. Epub 2015 Oct 17. Methods. 2016. PMID: 26484733 Free PMC article. Review. - A Narrative Review on Different Novel Machine Learning Techniques for Detecting Pathologies in Infants From Born Baby Cries.
Kumari P, Mahto K. Kumari P, et al. J Voice. 2024 May 6:S0892-1997(24)00077-8. doi: 10.1016/j.jvoice.2024.03.009. Online ahead of print. J Voice. 2024. PMID: 38714440 Review.
Cited by
- Efficient automatic 3D segmentation of cell nuclei for high-content screening.
Marzec M, Piórkowski A, Gertych A. Marzec M, et al. BMC Bioinformatics. 2022 May 31;23(1):203. doi: 10.1186/s12859-022-04737-4. BMC Bioinformatics. 2022. PMID: 35641922 Free PMC article. - A Machine Learning Assisted, Label-free, Non-invasive Approach for Somatic Reprogramming in Induced Pluripotent Stem Cell Colony Formation Detection and Prediction.
Fan K, Zhang S, Zhang Y, Lu J, Holcombe M, Zhang X. Fan K, et al. Sci Rep. 2017 Oct 18;7(1):13496. doi: 10.1038/s41598-017-13680-x. Sci Rep. 2017. PMID: 29044152 Free PMC article. - Rapid 3-D delineation of cell nuclei for high-content screening platforms.
Gertych A, Ma Z, Tajbakhsh J, Velásquez-Vacca A, Knudsen BS. Gertych A, et al. Comput Biol Med. 2016 Feb 1;69:328-38. doi: 10.1016/j.compbiomed.2015.04.025. Epub 2015 Apr 25. Comput Biol Med. 2016. PMID: 25982066 Free PMC article. - Dynamic proteomics of human protein level and localization across the cell cycle.
Farkash-Amar S, Eden E, Cohen A, Geva-Zatorsky N, Cohen L, Milo R, Sigal A, Danon T, Alon U. Farkash-Amar S, et al. PLoS One. 2012;7(11):e48722. doi: 10.1371/journal.pone.0048722. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144944 Free PMC article. - Cell cycle phase classification in 3D in vivo microscopy of Drosophila embryogenesis.
Du TH, Puah WC, Wasser M. Du TH, et al. BMC Bioinformatics. 2011;12 Suppl 13(Suppl 13):S18. doi: 10.1186/1471-2105-12-S13-S18. Epub 2011 Nov 30. BMC Bioinformatics. 2011. PMID: 22372955 Free PMC article.
References
- Yan J, Zhou X, Yang Q, Liu N, Cheng Q, Wong STC. An efficient system for optical microscopy cell image segmentation, tracking and cell phase identification. IEEE International symposium on Image Processing: 2006; Atlanta. 2006. pp. 1536–1537.
- Zhou X, Wong STC. High content cellular imaging for drug development. IEEE Signal Processing Magazine. 2006;23:170–174.
- Zhou X, Cao XH, Perlman Z, Wong STC. A computerized cellular imaging system for high content analysis in Monastrol suppressor screens. Journal of Biomedical Informatics. 2006;39:115–125. - PubMed
- Zhou X, Wong STC. Informatics challenges of high-throughput cellular and molecular microscopy. IEEE Signal Processing Magazine. 2006;23:63–72.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources