Functionally and spatially distinct modes of munc18-syntaxin 1 interaction - PubMed (original) (raw)
Functionally and spatially distinct modes of munc18-syntaxin 1 interaction
Colin Rickman et al. J Biol Chem. 2007.
Abstract
Eukaryotic membrane trafficking is a conserved process under tight temporal and spatial regulation in which the fusion of membranes is driven by the formation of the ternary SNARE complex. Syntaxin 1a, a core component of the exocytic SNARE complex in neurons and neuroendocrine cells, is regulated directly by munc18-1, its cognate Sec1p/munc18 (SM) protein. SM proteins show remarkable structural conservation throughout evolution, indicating a common binding mechanism and function. However, SM proteins possess disparate binding mechanisms and regulatory effects with munc18-1, the major brain isoform, classed as atypical in both its binding specificity and its mode. We now show that munc18-1 interacts with syntaxin 1a through two mechanistically distinct modes of binding, both in vitro and in living cells, in contrast to current models. Furthermore, these functionally divergent interactions occur at distinct cellular locations. These findings provide a molecular explanation for the multiple, spatially distinct roles of munc18-1.
Figures
Fig. 1
Syntaxin 1a can interact with munc18-1 through its N-terminus in vitro. A, Sequence alignment of syntaxin 1 homologues and Sed5p with similarity scored at each position (left panel). The highly conserved N-terminal region (a.a. 1- 28, right panel) is shown on a colour coded scale (yellow - identical, _cyan_-conserved, green - similar). B, Structural alignment of Sly1p (red) bound to an N-terminal peptide of Sed5p (green) (PDB: 1MQS(20)) and munc18-1 (pink) (PDB: 1EPU(39)). Highlighted on an enlarged view (right panel) are the absolutely conserved residues from panel a. C, Both Syx1-261 and Syx1-261 [open], immobilised on Sepharose beads, readily bind munc18-1. Native brain GSTs bound directly to the Sepharose resin. D, Truncation of the N-terminus of Syx1-261 [open] reduces its ability to bind munc18-1. N-terminal truncations of GST-Syx1-261 and GST-Syx1-261 [open] were immobilised on glutathione Sepharose beads and incubated with His6-munc18-1 containing bacterial lysate. Bound material was analysed by SDS PAGE and Coomassie staining. E, Circular dichroism spectra of Syx1-261, Syx1-261 (Δ6), Syx1-261 [open] and Syx1-261 [open] (Δ6). F, Measurement of the equilibrium dissociation constant for the syntaxin 1a-munc18-1 complex. GST-Syx1-261, or a mutant form, immobilised on Sepharose beads, was incubated in the presence of varying concentrations of munc18-1. Bound material was analysed by Western immunoblotting. Error bars represent S.E.M. (n=3).
Fig. 2
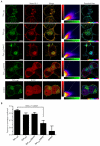
Munc18-1 colocalisation with syntaxin 1a in live cells is dependent on either closed form or N-terminal binding. A, Wild-type or mutant mCer-Syx (green), and EYFP-munc18-1 (red) were expressed in N2a cells and imaged by confocal laser scanning microscopy. The merge image shows areas of coincidence in yellow hues. The 2-D histogram represents the intensity for each channel in each voxel with a colour scale representing frequency. The residual map displays weighted residuals from the line fit to the histogram, thus indicating fluorescence channel covariance. The hue is from -1 to 1 with cyan corresponding to a zero residual. EYFP and unfused munc18-1 were used as a control. Scale bar: 2 μm. B, Combined covariance analysis of cerulean-Syx and EYFP-munc18-1 in N2a cells. Mean Pearson’s coefficients are shown (n>4).
Fig. 3
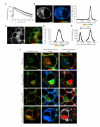
Different binding modes of munc18-1 - syntaxin 1a are spatially distinct. A, The excited state fluorescence decay of cerulean-Syx1-288 in the absence of an energy acceptor followed a mono-exponential decay, as previously described for cerulean(40) (dark circles). When co-expressed with EYFP-munc18-1, the cerulean-Syx1-288 decay data no longer fit to a single exponential, but were well described by a bi-exponential decay function (light grey circles). B, Cerulean-Syx1-288 fluorescence, in the absence of EYFP-munc18-1, exhibited a plasma membrane and intracellular membrane distribution (left panel) The colour scale in the “FLIM map” represents the fluorescence lifetime and brightness represents intensity (centre panel). The fluorescence lifetime values were plotted as a frequency distribution histogram (right panel) with a single fluorescence lifetime of 2288 ± 40 ps (mean ± S.E.M., n=18). C, The intensity localisation of cerulean-Syx1-288, in the presence of EYFP-munc18-1, was unaltered (left panel), but the FLIM map reveals a quenched fluorescence lifetime (right panel). The colours in this map represent the weighted mean of the two lifetimes for each pixel, one identical to the non-FRET lifetime (2288 ps) and one significantly shorter (680 ± 34 ps mean ± S.E.M; p<0.0001, n = 12). These data are plotted as the weighted mean (significantly different to the non-FRET distribution) (D) and as separate fluorescence lifetimes (E), representing the non-FRET and the FRET component contained in each pixel. The amplitudes of these components (which combine to 100%) represent the relative proportion of each lifetime. F, These approaches were applied to each syntaxin 1a mutant, in addition calculating the lifetime amplitude ratio of FRET:non-FRET amplitudes in each pixel. All data treatments were identical. Pixels containing only non-FRET values appear in greyscale. Amplitude ratio scale: zero (no FRET component, greyscale), 0.1-0.66 (red), 0.66-1.33 (green), 1.33-2.5 (blue). For all panels (_A_-F): scale bars represent 2 μm and fluorescence lifetime color scales 1250 ps (red)-2250 ps (blue).
Fig. 4
Munc18-1 can remain associated with syntaxin 1a through the SNARE assembly pathway. A, Munc18-1 in complex with Syx1-261 (left panel), Syx7-261 (Δ6) (centre panel) or Syx1-261 [open] (right panel) was incubated with immobilised SNAP-25 in the presence or absence of synaptobrevin (Syb). B, As a control munc18-1 alone was incubated with immobilised SNAP-25. C, Proposed model for munc18-1 interaction with syntaxin 1a. An undefined factor releases syntaxin from its closed state on the plasma membrane permitting binary and ternary SNARE complex formation. Munc18-1 can remain bound to the N-terminus of syntaxin 1a through this process. It was not possible to purify a Syx7-261[open](Δ6)/munc18-1 complex for use in this binding assay because of the decreased affinity of Syx7-261[open](Δ6) for munc18-1.
Similar articles
- Syntaxin N-terminal peptide motif is an initiation factor for the assembly of the SNARE-Sec1/Munc18 membrane fusion complex.
Rathore SS, Bend EG, Yu H, Hammarlund M, Jorgensen EM, Shen J. Rathore SS, et al. Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22399-406. doi: 10.1073/pnas.1012997108. Epub 2010 Dec 7. Proc Natl Acad Sci U S A. 2010. PMID: 21139055 Free PMC article. - Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus.
Khvotchev M, Dulubova I, Sun J, Dai H, Rizo J, Südhof TC. Khvotchev M, et al. J Neurosci. 2007 Nov 7;27(45):12147-55. doi: 10.1523/JNEUROSCI.3655-07.2007. J Neurosci. 2007. PMID: 17989281 Free PMC article. - S-nitrosylation of syntaxin 1 at Cys(145) is a regulatory switch controlling Munc18-1 binding.
Palmer ZJ, Duncan RR, Johnson JR, Lian LY, Mello LV, Booth D, Barclay JW, Graham ME, Burgoyne RD, Prior IA, Morgan A. Palmer ZJ, et al. Biochem J. 2008 Aug 1;413(3):479-91. doi: 10.1042/BJ20080069. Biochem J. 2008. PMID: 18452404 - Munc18-1 as a key regulator of neurosecretion.
Han GA, Malintan NT, Collins BM, Meunier FA, Sugita S. Han GA, et al. J Neurochem. 2010 Oct;115(1):1-10. doi: 10.1111/j.1471-4159.2010.06900.x. J Neurochem. 2010. PMID: 20681955 Review. - Neuronal SNARE complex assembly guided by Munc18-1 and Munc13-1.
Wang S, Ma C. Wang S, et al. FEBS Open Bio. 2022 Nov;12(11):1939-1957. doi: 10.1002/2211-5463.13394. Epub 2022 Mar 22. FEBS Open Bio. 2022. PMID: 35278279 Free PMC article. Review.
Cited by
- Rab-3 and unc-18 interactions in alcohol sensitivity are distinct from synaptic transmission.
Johnson JR, Kashyap S, Rankin K, Barclay JW. Johnson JR, et al. PLoS One. 2013 Nov 14;8(11):e81117. doi: 10.1371/journal.pone.0081117. eCollection 2013. PLoS One. 2013. PMID: 24244732 Free PMC article. - Dual roles of Munc18-1 rely on distinct binding modes of the central cavity with Stx1A and SNARE complex.
Shi L, Kümmel D, Coleman J, Melia TJ, Giraudo CG. Shi L, et al. Mol Biol Cell. 2011 Nov;22(21):4150-60. doi: 10.1091/mbc.E11-02-0150. Epub 2011 Sep 7. Mol Biol Cell. 2011. PMID: 21900493 Free PMC article. - Imaging large cohorts of single ion channels and their activity.
Hiersemenzel K, Brown ER, Duncan RR. Hiersemenzel K, et al. Front Endocrinol (Lausanne). 2013 Sep 4;4:114. doi: 10.3389/fendo.2013.00114. Front Endocrinol (Lausanne). 2013. PMID: 24027557 Free PMC article. Review. - The Munc18-1 domain 3a hinge-loop controls syntaxin-1A nanodomain assembly and engagement with the SNARE complex during secretory vesicle priming.
Kasula R, Chai YJ, Bademosi AT, Harper CB, Gormal RS, Morrow IC, Hosy E, Collins BM, Choquet D, Papadopulos A, Meunier FA. Kasula R, et al. J Cell Biol. 2016 Sep 26;214(7):847-58. doi: 10.1083/jcb.201508118. Epub 2016 Sep 19. J Cell Biol. 2016. PMID: 27646276 Free PMC article. - Munc18-1 mutations that strongly impair SNARE-complex binding support normal synaptic transmission.
Meijer M, Burkhardt P, de Wit H, Toonen RF, Fasshauer D, Verhage M. Meijer M, et al. EMBO J. 2012 May 2;31(9):2156-68. doi: 10.1038/emboj.2012.72. Epub 2012 Mar 23. EMBO J. 2012. PMID: 22446389 Free PMC article.
References
- Burgoyne RD, Morgan A. Physiol Rev. 2003;83(2):581–632. - PubMed
- Rizo J, Sudhof TC. Nat Rev Neurosci. 2002;3(8):641–653. - PubMed
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Nature. 1998;395(6700):347–353. - PubMed
- Harrison SD, Broadie K, van de Goor J, Rubin GM. Neuron. 1994;13(3):555–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources