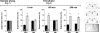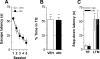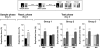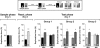On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory - PubMed (original) (raw)
On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory
Janine I Rossato et al. Learn Mem. 2007 January-February.
Abstract
Upon retrieval, consolidated memories are again rendered vulnerable to the action of metabolic blockers, notably protein synthesis inhibitors. This has led to the hypothesis that memories are reconsolidated at the time of retrieval, and that this depends on protein synthesis. Ample evidence indicates that the hippocampus plays a key role both in the consolidation and reconsolidation of different memories. Despite this fact, at present there are no studies about the consequences of hippocampal protein synthesis inhibition in the storage and post-retrieval persistence of object recognition memory. Here we report that infusion of the protein synthesis inhibitor anisomycin in the dorsal CA1 region immediately or 180 min but not 360 min after training impairs consolidation of long-term object recognition memory without affecting short-term memory, exploratory behavior, anxiety state, or hippocampal functionality. When given into CA1 after memory reactivation in the presence of familiar objects, ANI did not affect further retention. However, when administered into CA1 immediately after exposing animals to a novel and a familiar object, ANI impaired memory of both of them. The amnesic effect of ANI was long-lasting, did not happen after exposure to two novel objects, following exploration of the context alone, or in the absence of specific stimuli, suggesting that it was not reversible but was contingent on the reactivation of the consolidated trace in the presence of a salient, behaviorally relevant novel cue. Our results indicate that hippocampal protein synthesis is required during a limited post-training time window for consolidation of object recognition memory and show that the hippocampus is engaged during reconsolidation of this type of memory, maybe accruing new information into the original trace.
Figures
Figure 1.
Hippocampal protein synthesis is required during a restricted post-training time window for consolidation of object recognition memory. On day 1 (sample phase) rats (n = 74) were exposed to two different objects (A and B) for 5 min and at different times after that (0, 180, or 360 min) received bilateral infusions (0.8 μL/side) of vehicle (VEH; saline) or anisomycin (ANI; 160 μg/side) in the CA1 region of the dorsal hippocampus. On day 2 (Test phase) animals were exposed to a familiar (A) and a novel object (C) for an additional 5 min. Data are presented as mean (±SEM) of the percentage of time exploring a particular object over the total time of object exploration. ***P < 0.0005, **P < 0.005 in Student’s _t_-test; n = 10–15 per group. Note that animals that received ANI immediately or 180 min after training spent the same amount of time exploring objects A and C during the test phase (Day 2; 0 min—ANI and 180 min—ANI) indicating that recognition memory was impaired. (Right) Schematic drawings taken from the atlas of Paxinos and Watson (1986) and representative photomicrograph showing the location of the infusion cannulae tips in dorsal CA1 for the animals that received post-training ANI.
Figure 2.
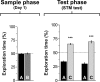
Post-training inhibition of hippocampal protein synthesis does not affect short-term object recognition memory. Rats (n = 16) were exposed to two different objects (A and B) for 5 min and immediately after that received bilateral infusions (0.8 μL/side) of vehicle (VEH; saline) or anisomycin (ANI; 160 μg/side) in the CA1 region of the dorsal hippocampus. Three hours later (Test phase/STM test) animals were exposed to a familiar object (A) and a novel object (C) for an additional 5 min. Data are presented as mean (±SEM) of the percentage of time exploring a particular object over the total time of object exploration. ***P < 0.0005 in Student’s _t_-test; n = 8 per group.
Figure 3.
Inhibition of hippocampal protein synthesis 24 h before training does not affect acquisition or retention of hippocampal-dependent memories. (A) Mean escape latency during the 5 d of acquisition of spatial learning for rats (n = 16) given anisomycin (160 μg/side; ANI; black circles) or vehicle (VEH; white circles) in the CA1 region of the dorsal hippocampus 24 h before the first training session. Data are presented in blocks of eight trials as mean ±SEM. (B) Percentage of time spent in the target quadrant (TQ) during a 60-sec probe test carried out 24 h after the fifth training day for the rats shown in A. Data are presented as mean ±SEM. **P < 0.005 in one-sample Student’s _t_-test (reference value = 25%) (C) Rats with infusion cannulae implanted in the CA1 region of the dorsal hippocampus (n = 18) received intra-CA1 infusions of anisomycin (160 μg/side; ANI) or vehicle (VEH) and 24 h afterward were trained in a one-trial, step-down inhibitory avoidance task. Data are presented as median ± interquartile range of step-down latency in the training session (TR) and during a long-term memory (LTM) retention test session performed 24 h post-training. **P < 0.005 vs. the respective TR value in a Mann-Whitney U-test.
Figure 4.
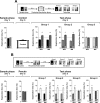
Inhibition of hippocampal protein synthesis after a reactivation session involving exposition to familiar objects does not affect further retention of recognition memory. (A) Rats with infusion cannulae implanted in the CA1 region of the dorsal hippocampus (n = 64) were exposed to two objects (A and B) for 5 min (Sample phase, Day 1). Twenty-four hours later the animals were re-exposed for an additional 5 min to the same two objects to reactivate the memory trace (Reactivation phase, Day 2), and at different times after that (0, 180, or 360 min) received bilateral intra-CA1 infusions of anisomycin (160 μg/side; ANI) or vehicle (VEH). Retention was assessed 24 h later by exposing animals to the familiar object A plus a novel object C (Test phase, Day 3). Note that regardless of the treatment and the moment of infusion, animals spent more time exploring the novel than the familiar object, indicating that memory had been preserved. ***P < 0.0005, **P < 0.005 in Student’s _t_-test; n = 10–13 per group. (B) Animals (n = 20) were treated as in A except that the reactivation session was just 2 min instead of 5 min long and rats received VEH or ANI (160 μg/side) immediately after the reactivation phase. **P < 0.005, *P < 0.05 in Student’s _t_-test; n = 10 per group. (C) Animals (n = 22) were treated as in A except that the 5 min reactivation phase was performed 5 d instead of 1 d after training and rats received VEH or ANI (160 μg/side) immediately after the reactivation phase. In all cases data are presented as mean (±SEM) of the percentage of time exploring a particular object over the total time of object exploration. ***P < 0.0005, **P < 0.005 in Student’s _t_-test; n = 11 per group.
Figure 5.
Inhibition of hippocampal protein synthesis after a reactivation session involving exposition to a novel and a familiar object impairs memory of these two objects but does not affect that of a familiar object not presented during the reactivation phase. Rats with infusion cannulae implanted in the CA1 region of the dorsal hippocampus (n = 63) were exposed to two objects (A and B) for 5 min (Sample phase; Day 1). Twenty-four hours later the animals were exposed to familiar object A plus a novel object (C) (Reactivation phase; Day 2). Rats were randomly assigned to one out of three different groups and immediately after that received bilateral intra-CA1 infusions of either vehicle (VEH) or anisomycin (160 μg/side; ANI). Twenty-four hours later animals were submitted to a 5-min-long test phase (Test phase; Day 3) in the presence of different combinations of objects, as follows: (Group 1) Object A + Object D; (Group 2) Object B + Object D; (Group 3) Object C + Object D, where D was a novel object. Note that ANI impaired retention of the memory for the novel object C and also for the familiar object A (which was presented during the reactivation session) but spared memory for familiar object B, to which animals were not exposed during the reactivation phase. Data are presented as mean (±SEM) of the percentage of time exploring a particular object over the total time of object exploration. ***P < 0.0005, **P < 0.005 in Student’s _t_-test; n = 9–12 per group. (Top) Schematic representation of the behavioral protocol used.
Figure 6.
The time elapsed between reactivation and test sessions had no effect on the amnesia caused by the post-reactivation infusion of ANI in the CA1 region of the hippocampus. Rats with infusion cannulae implanted in the CA1 region of the dorsal hippocampus (n = 59) were exposed to two objects (A and B) for 5 min (Sample phase; Day 1). Five days later the animals were exposed to familiar object A plus a novel object (C) (Reactivation phase; Day 6). Rats were randomly assigned to one out of three different groups and immediately after that received bilateral intra-CA1 infusions of either vehicle (VEH) or anisomycin (160 μg/side; ANI). Twenty-four hours later animals were submitted to a 5-min-long test phase (Test phase; Day 7) in the presence of different combinations of objects, as follows: (Group 1) Object A + Object D; (Group 2) Object B + Object D; (Group 3) Object C + Object D, where D was a novel object. Note that ANI impaired retention of the memory for the novel object C and also for the familiar object A (which was presented during the reactivation session) but spared memory for familiar object B, to which animals were not exposed during the reactivation phase. Data are presented as mean (±SEM) of the percentage of time exploring a particular object over the total time of object exploration. ***P < 0.0005, **P < 0.005 in Student’s _t_-test; n = 9–10 per group. (Top) Schematic representation of the behavioral protocol used.
Figure 7.
Inhibition of hippocampal protein synthesis 24 h after training in the absence of a behaviorally relevant event does not affect retention of object recognition memory. Rats with infusion cannulae implanted in the CA1 region of the dorsal hippocampus (n = 60) were exposed to two objects (A and B) for 5 min (Sample phase; Day 1). Twenty-four hours later animals were randomly assigned to one out of three different groups, were given bilateral intra-CA1 infusions of either vehicle (VEH) or anisomycin (160 μg/side; ANI), and after that they were returned to their home cages without receiving any specific stimuli. Twenty-four hours later animals were submitted to a 5-min-long test phase (Test phase; Day 3) in the presence of different combinations of objects, as follows: (Group 1) Object A + Object D; (Group 2) Object B + Object D; (Group 3) Object C + Object D, where C and D were novel objects. Data are presented as mean (±SEM) of the percentage of time exploring a particular object over the total time of object exploration. ***P < 0.0005, **P < 0.005 in Student’s _t_-test; n = 10 per group. (Top) Schematic representation of the behavioral protocol used.
Figure 8.
Inhibition of hippocampal protein synthesis after exposure to the training box context alone or following a pseudoreactivation session involving exposition to two novel objects does not affect the original recognition memory. (A) Rats with infusion cannulae implanted in the CA1 region of the dorsal hippocampus (n = 62) were exposed to two objects (A and B) for 5 min (Sample phase, Day 1). Twenty-four hours later the animals were left to freely explore the open field arena in the absence (Contextual Reactivation phase; Day 2). Rats were randomly assigned to one out of three different groups and immediately after that received bilateral intra-CA1 infusions of either vehicle (VEH) or anisomycin (160 μg/side; ANI). Twenty-four hours later animals were submitted to a 5-min-long test phase (Test phase; Day 3) in the presence of different combinations of objects, as follows: (Group 1) Object A + Object D; (Group 2) Object B + Object D; (Group 3) Object C + Object D, where C and D were novel objects. ***P < 0.0005, **P < 0.005 in Student’s _t_-test; n = 10–12 per group. (Top) Schematic representation of the behavioral protocol used. (B) Rats with infusion cannulae implanted in the CA1 region of the dorsal hippocampus (n = 77) were exposed to two objects (A and B) for 5 min (Sample phase; Day 1). Twenty-four hours later the animals were exposed to two novel objects D and E (Pseudoreactivation phase; Day 2). Rats were randomly assigned to one out of four different groups and immediately after that received bilateral intra-CA1 infusions of either vehicle (VEH) or anisomycin (160 μg/side; ANI). Twenty-four hours later animals were submitted to a 5-min-long test phase (Test phase; Day 3) in the presence of different combinations of objects, as follows: (Group 1) Object A + Object F; (Group 2) Object B + Object F; (Group 3) Object D + Object F; (Group 4) Object E + Object F, where F was a novel object. Note that ANI impaired retention of the memory for the novel objects D and E (which were presented during the Pseudoreactivation session) but spared memory for familiar objects A and B, to which animals were only exposed during the sample phase. Data are presented as mean (±SEM) of the percentage of time exploring a particular object over the total time of object exploration. ***P < 0.0005, **P < 0.005 in Student’s _t_-test; n = 8–10 per group. (Top) Schematic representation of the behavioral protocol used.
Similar articles
- On the participation of mTOR in recognition memory.
Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. Myskiw JC, et al. Neurobiol Learn Mem. 2008 Mar;89(3):338-51. doi: 10.1016/j.nlm.2007.10.002. Epub 2007 Nov 26. Neurobiol Learn Mem. 2008. PMID: 18039584 - State-dependent effect of dopamine D₁/D₅ receptors inactivation on memory destabilization and reconsolidation.
Rossato JI, Köhler CA, Radiske A, Lima RH, Bevilaqua LR, Cammarota M. Rossato JI, et al. Behav Brain Res. 2015 May 15;285:194-9. doi: 10.1016/j.bbr.2014.09.009. Epub 2014 Sep 16. Behav Brain Res. 2015. PMID: 25219363 - The relationship between protein synthesis and protein degradation in object recognition memory.
Furini CR, Myskiw Jde C, Schmidt BE, Zinn CG, Peixoto PB, Pereira LD, Izquierdo I. Furini CR, et al. Behav Brain Res. 2015 Nov 1;294:17-24. doi: 10.1016/j.bbr.2015.07.038. Epub 2015 Jul 19. Behav Brain Res. 2015. PMID: 26200717 - Reconsolidation and the fate of consolidated memories.
Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. Bevilaqua LR, et al. Neurotox Res. 2008 Dec;14(4):353-8. doi: 10.1007/BF03033859. Neurotox Res. 2008. PMID: 19073438 Review. - Relevance of ERK1/2 Post-retrieval Participation on Memory Processes: Insights in Their Particular Role on Reconsolidation and Persistence of Memories.
Krawczyk MC, Millan J, Blake MG, Feld M, Boccia MM. Krawczyk MC, et al. Front Mol Neurosci. 2019 Apr 17;12:95. doi: 10.3389/fnmol.2019.00095. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31057366 Free PMC article. Review.
Cited by
- A short-term memory trace persists for days in the mouse hippocampus.
Wally ME, Nomoto M, Abdou K, Murayama E, Inokuchi K. Wally ME, et al. Commun Biol. 2022 Nov 3;5(1):1168. doi: 10.1038/s42003-022-04167-1. Commun Biol. 2022. PMID: 36329137 Free PMC article. - Forebrain glucocorticoid receptor overexpression increases environmental reactivity and produces a stress-induced spatial discrimination deficit.
Hebda-Bauer EK, Pletsch A, Darwish H, Fentress H, Simmons TA, Wei Q, Watson SJ, Akil H. Hebda-Bauer EK, et al. Neuroscience. 2010 Aug 25;169(2):645-53. doi: 10.1016/j.neuroscience.2010.05.033. Epub 2010 May 31. Neuroscience. 2010. PMID: 20562006 Free PMC article. - On the Involvement of BDNF Signaling in Memory Reconsolidation.
Gonzalez MC, Radiske A, Cammarota M. Gonzalez MC, et al. Front Cell Neurosci. 2019 Aug 22;13:383. doi: 10.3389/fncel.2019.00383. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31507380 Free PMC article. Review. - Distinct contributions of the hippocampus and medial prefrontal cortex to the "what-where-when" components of episodic-like memory in mice.
DeVito LM, Eichenbaum H. DeVito LM, et al. Behav Brain Res. 2010 Dec 31;215(2):318-25. doi: 10.1016/j.bbr.2009.09.014. Epub 2009 Sep 17. Behav Brain Res. 2010. PMID: 19766146 Free PMC article. - The Impact of Probiotic Supplementation on Cognitive, Pathological and Metabolic Markers in a Transgenic Mouse Model of Alzheimer's Disease.
Webberley TS, Masetti G, Bevan RJ, Kerry-Smith J, Jack AA, Michael DR, Thomas S, Glymenaki M, Li J, McDonald JAK, John D, Morgan JE, Marchesi JR, Good MA, Plummer SF, Hughes TR. Webberley TS, et al. Front Neurosci. 2022 May 24;16:843105. doi: 10.3389/fnins.2022.843105. eCollection 2022. Front Neurosci. 2022. PMID: 35685773 Free PMC article.
References
- Aggleton J.P., Brown M.W. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q. J. Exp. Psychol. B. 2005;58:218–233. - PubMed
- Akirav I., Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb. Cortex. 2006;16:1759–1765. - PubMed
- Alberini C.M. Mechanisms of memory stabilization: Are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. - PubMed
- Barraco D.A., Lovell K.L., Eisenstein E.M. Effects of cycloheximide and puromycin on learning and retention in the cockroach, P. americana. Pharmacol. Biochem. Behav. 1981;15:489–494. - PubMed
- Bevilaqua L.R., Medina J.H., Izquierdo I., Cammarota M. Memory consolidation induces N-methyl-d-aspartic acid-receptor- and Ca2+/calmodulin-dependent protein kinase II-dependent modifications in α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor properties. Neuroscience. 2005;136:397–403. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous