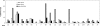Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1 - PubMed (original) (raw)
Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1
William G Roach et al. Biochem J. 2007.
Abstract
Insulin stimulation of the trafficking of the glucose transporter GLUT4 to the plasma membrane is controlled in part by the phosphorylation of the Rab GAP (GTPase-activating protein) AS160 (also known as Tbc1d4). Considerable evidence indicates that the phosphorylation of this protein by Akt (protein kinase B) leads to suppression of its GAP activity and results in the elevation of the GTP form of a critical Rab. The present study examines a similar Rab GAP, Tbc1d1, about which very little is known. We found that the Rab specificity of the Tbc1d1 GAP domain is identical with that of AS160. Ectopic expression of Tbc1d1 in 3T3-L1 adipocytes blocked insulin-stimulated GLUT4 translocation to the plasma membrane, whereas a point mutant with an inactive GAP domain had no effect. Insulin treatment led to the phosphorylation of Tbc1d1 on an Akt site that is conserved between Tbc1d1 and AS160. These results show that Tbc1d1 regulates GLUT4 translocation through its GAP activity, and is a likely Akt substrate. An allele of Tbc1d1 in which Arg(125) is replaced by tryptophan has very recently been implicated in susceptibility to obesity by genetic analysis. We found that this form of Tbc1d1 also inhibited GLUT4 translocation and that this effect also required a functional GAP domain.
Figures
Figure 1. Schematic representations of mouse Tbc1d1 and AS160
The amino acid sequences used are NCBI gi 37538012 for mouse Tbc1d1 and NCBI gi 67462068 for mouse AS160. Tbc1d1 and AS160 are proteins of 1255 and 1307 amino acids respectively. The two PTB domains and the GAP domains are those predicted by analysis with the Pfam program (
). The wavy line at the border of a domain indicates that the domain alignment is incomplete at that end of the domain. SV designates the section deleted from the shorter slice variant. CM designates the calmodulin-binding motif. The sites of insulin-stimulated phosphorylation of AS160, most likely by the protein kinase Akt, are numbered, as are the two such sites conserved in Tbc1d1. The numbers in parentheses are the numbers of the same Akt sites in the human versions of Tbc1d1 (NCBI gi 54887445) and AS160 (NCBI gi 114688046). Thr590 (Thr596 in humans) of Tbc1d1 corresponds to Thr649 (Thr642 in humans) of AS160; Ser501 (Ser507 in humans) of Tbc1d1 corresponds to Ser577 (Ser570 in humans) of AS160.
Figure 2. Activity of Tbc1d1 GAP domain against 20 Rab proteins
The GAP assay was carried out as described in the Experimental section. The data show the increase in the percentage of the total radioactivity in the GDP form between 0 and 15 min for the Rab alone, the Rab plus the GAP domain, and the Rab plus the GAP domain with the R854K mutation. In the case of Rabs 3A and 5A the data are for 6 min and 10 min respectively, rather than 15 min, due to the higher intrinsic GTPase activity of these Rabs. These data were taken from the time courses of GDP formation over the period from 0 to 30 min, with a second time point taken at 30 min (except for Rabs 3A and 5A where the second time point was at 12 or 20 min respectively); the results at the longer time point again showed significant GAP activity against only Rabs 2A, 8A, 8B, 10 and 14. Separate replicate determinations of the times courses for Rabs 3A, 10 and 14 yielded the same results for these Rabs as shown here.
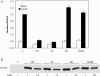
Figure 3. Effects of Tbc1d1 on GLUT4 translocation
(A) 3T3-L1 adipocytes were transfected with HA–GLUT4–GFP and vector (V), wild-type Tbc1d1 (WT) or one of the mutant forms of Tbc1d1 (TA, T596A; RK, R854K; TA/RK, T596A/R854K). The relative amount of HA–GLUT4–GFP at the cell surface in basal and insulin-stimulated 3T3-L1 adipocytes was measured using the single-cell fluorescence assay, as described in the Experimental section. Results are means±S.E.M. for three separate experiments. The values in each experiment were normalized to a value of 1.0 for the vector in the insulin state. (B) SDS lysates were made of the transfected cells described in (A) and immunoblotted for the FLAG epitope. The 1× load per lane was 5 μg of protein.
Figure 4. Phosphorylation on Thr596 of Tbc1d1
(A) HEK-293 cells were transfected with vector (Vec) or the various forms of Tbc1d1 and treated with wortmannin (W) for or insulin (I), as described in the Experimental section. SDS lysates were prepared and immunoblotted for phosphorylation with the anti-PAS antibody, for Tbc1d1 with antibody against its FLAG epitope tag, and for Akt activation with antibody for the Ser473 phosphorylation site on Akt. (B) 3T3-L1 adipocytes were transfected with vector or the various forms of Tbc1d1 and treated with 160 nM insulin for 10 min (I) or left in the basal state (B). SDS/non-ionic detergent lysates were prepared and the FLAG-tagged Tbc1d1 was immunoprecipitated, as described in the Experimental section. The immunoprecipitate was blotted with the anti-PAS antibody and with anti-FLAG. SDS samples of the total cell lysates were immunoblotted for activation of Akt as in (A). The experiments in (A) and (B) were repeated and gave similar results.
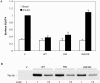
Figure 5. Effects of the R125W mutation on Tbc1d1 inhibition of GLUT4 translocation
(A) 3T3-L1 adipocytes were transfected with HA–GLUT4–GFP and vector (V), wild-type Tbc1d1 (WT), the R125W mutant (RW), or the R125W/R854K mutant (RW/RK), and assayed for GLUT4 at the cell surface, as described in the Experimental section and the legend to Figure 3. Results are means±S.E.M. for four experiments, normalized to a value of 1.0 for the vector plus insulin. (B) SDS lysates of the transfected cells were immunoblotted for the FLAG epitope. The 1× load was 5 μg of protein.
Comment in
- Thrifty Tbc1d1 and Tbc1d4 proteins link signalling and membrane trafficking pathways.
Koumanov F, Holman GD. Koumanov F, et al. Biochem J. 2007 Apr 15;403(2):e9-11. doi: 10.1042/BJ20070271. Biochem J. 2007. PMID: 17376030 Free PMC article.
Similar articles
- Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation.
Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Chavez JA, et al. J Biol Chem. 2008 Apr 4;283(14):9187-95. doi: 10.1074/jbc.M708934200. Epub 2008 Feb 7. J Biol Chem. 2008. PMID: 18258599 Free PMC article. - Insulin-stimulated phosphorylation of the Rab GTPase-activating protein TBC1D1 regulates GLUT4 translocation.
Peck GR, Chavez JA, Roach WG, Budnik BA, Lane WS, Karlsson HK, Zierath JR, Lienhard GE. Peck GR, et al. J Biol Chem. 2009 Oct 30;284(44):30016-23. doi: 10.1074/jbc.M109.035568. Epub 2009 Sep 9. J Biol Chem. 2009. PMID: 19740738 Free PMC article. - Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation.
Ishikura S, Klip A. Ishikura S, et al. Am J Physiol Cell Physiol. 2008 Oct;295(4):C1016-25. doi: 10.1152/ajpcell.00277.2008. Epub 2008 Aug 13. Am J Physiol Cell Physiol. 2008. PMID: 18701652 - Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic.
Sakamoto K, Holman GD. Sakamoto K, et al. Am J Physiol Endocrinol Metab. 2008 Jul;295(1):E29-37. doi: 10.1152/ajpendo.90331.2008. Epub 2008 May 13. Am J Physiol Endocrinol Metab. 2008. PMID: 18477703 Free PMC article. Review. - Regulation of RabGAPs involved in insulin action.
Mafakheri S, Chadt A, Al-Hasani H. Mafakheri S, et al. Biochem Soc Trans. 2018 Jun 19;46(3):683-690. doi: 10.1042/BST20170479. Epub 2018 May 21. Biochem Soc Trans. 2018. PMID: 29784647 Review.
Cited by
- AS160 is a lipid-responsive regulator of cardiac Ca2+ homeostasis by controlling lysophosphatidylinositol metabolism and signaling.
Su S, Quan C, Chen Q, Wang R, Du Q, Zhu S, Li M, Yang X, Rong P, Chen J, Bai Y, Zheng W, Feng W, Liu M, Xie B, Ouyang K, Shi YS, Lan F, Zhang X, Xiao R, Chen X, Wang HY, Chen S. Su S, et al. Nat Commun. 2024 Nov 6;15(1):9602. doi: 10.1038/s41467-024-54031-5. Nat Commun. 2024. PMID: 39505896 Free PMC article. - TBC1D1 is an energy-responsive polarization regulator of macrophages via governing ROS production in obesity.
Wang Q, Rong P, Zhang W, Yang X, Chen L, Cao Y, Liu M, Feng W, Ouyang Q, Chen Q, Li H, Liang H, Meng F, Wang HY, Chen S. Wang Q, et al. Sci China Life Sci. 2024 Sep;67(9):1899-1914. doi: 10.1007/s11427-024-2628-1. Epub 2024 Jun 14. Sci China Life Sci. 2024. PMID: 38902450 - Phosphorylation of AS160-serine 704 is not essential for exercise-increase in insulin-stimulated glucose uptake by skeletal muscles from female or male rats.
Wang H, Kwak SE, Zheng A, Arias EB, Pan X, Duan D, Cartee GD. Wang H, et al. Am J Physiol Endocrinol Metab. 2024 Jun 1;326(6):E807-E818. doi: 10.1152/ajpendo.00010.2024. Epub 2024 Apr 24. Am J Physiol Endocrinol Metab. 2024. PMID: 38656130 - Revving the engine: PKB/AKT as a key regulator of cellular glucose metabolism.
Li X, Hu S, Cai Y, Liu X, Luo J, Wu T. Li X, et al. Front Physiol. 2024 Jan 8;14:1320964. doi: 10.3389/fphys.2023.1320964. eCollection 2023. Front Physiol. 2024. PMID: 38264327 Free PMC article. Review. - Effects of swimming training in hot and cold temperatures combined with cinnamon supplementation on HbA1C levels, TBC1D1, and TBC1D4 in diabetic rats.
Tayebi SM, Nouri AH, Tartibian B, Ahmadabadi S, Basereh A, Jamhiri I. Tayebi SM, et al. Nutr Diabetes. 2024 Jan 10;14(1):1. doi: 10.1038/s41387-023-00256-0. Nutr Diabetes. 2024. PMID: 38195613 Free PMC article.
References
- Watson R. T., Kanzaki M., Pessin J. E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. - PubMed
- Thong F. S. L., Dugani C. B., Klip A. Turning signals on and off: GLUT4 traffic in the insulin signaling highway. Physiology. 2005;20:271–284. - PubMed
- Kane S., Sano H., Liu S. C., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. A method to identify serine kinase substrates: Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 2002;277:22115–22118. - PubMed
- Sano H., Kane S., Sano E., Mîinea C. P., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003;278:14599–14602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK25336/DK/NIDDK NIH HHS/United States
- R56 DK025336/DK/NIDDK NIH HHS/United States
- R01 DK025336/DK/NIDDK NIH HHS/United States
- DK07508/DK/NIDDK NIH HHS/United States
- T32 DK007508/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous