Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages - PubMed (original) (raw)
Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages
Yi Yang et al. Immunity. 2007 Feb.
Abstract
gp96 is an endoplasmic reticulum chaperone for cell-surface Toll-like receptors (TLRs). Little is known about its roles in chaperoning other TLRs or in the biology of macrophage in vivo. We generated a macrophage-specific gp96-deficient mouse. Despite normal development and activation by interferon-gamma, tumor necrosis factor-alpha, and interleukin-1beta, the mutant macrophages failed to respond to ligands of both cell-surface and intracellular TLRs including TLR2, TLR4, TLR5, TLR7, and TLR9. Furthermore, we found that TLR4 and TLR9 preferentially interacted with a super-glycosylated gp96 species. The categorical loss of TLRs in gp96-deficient macrophages operationally created a conditional and cell-specific TLR null mouse. These mice were resistant to endotoxin shock but were highly susceptible to Listeria monocytogenes. Our results demonstrate that gp96 is the master chaperone for TLRs and that macrophages, but not other myeloid cells, are the dominant source of proinflammatory cytokines during endotoxemia and Listeria infections.
Figures
Figure 1. Generation of a Conditional gp96-Deficient Mouse
(A) The scheme of targeting Hsp90b1 locus and expected sequence of Hsp90b1 loci after cre-mediated recombination. Neo, neomycin phosophotransferase; tk, thymidine kinase; X, XbaI; E, exon. (B) Confirmation of targeted Hsp90b1 allele by Southern blot with probe indicated in (A). (C) Flow cytometric analysis of GFP activity in various subsets of splenocytes of LysMcre+ Z/EG mice. Percent of GFP+ cells is indicated. (D) IB analysis of gp96 in BMDM and pMϕ from LysMcre+/–Hsp90b1flox/– and control mice. (E) Quantification of gp96 in splenic CD11b+ cells, BMDM, as well as pMf by intracellular staining (gp96, open histogram; isotype control, shaded histogram). Number represents the mean fluorescence intensity of gp96 stain. (F) Intracellular analysis of gp96 in different lineages of hematopoietic cells in the spleens of KO (open histogram with solid line) and WT (dark gray-shaded histogram) mice. Histograms at the far left represent isotype controls that are superimposable between KO (open histogram with dotted line) and WT (light gray-shaded histogram) mice. More than three experiments were done with the similar results.
Figure 2. Normal Hematopoiesis, Differentiation, and Cytokine-Mediated Activation of Mϕ in LysMcre+/–Hsp90b1flox/– Mice
(A) Thymocytes of KO or control littermates were stained for CD4 and CD8, followed by flow cytometric analysis. (B) Analysis of splenocytes by flow cytometry. RBC-depleted and collagenase-treated single-cell suspensions of splenocytes were stained with a combination of either Ab against TCR Vβ and B220 or Ab against CD11b and Gr-1. (C) Analysis of splenic DC based on expression of CD11c and a plasmacytoid DC marker, pDCA-1. CD11c+ DC were then further analyzed for the expression of CD4 and CD8. Numbers represent percent of cells in each quadrant or the marked area over the total cellular populations. (D) Immunofluorescence microscopy of WT or gp96 KO spleens. WT and gp96 KO mice were sacrificed. 5 mμ cryosections were prepared and stained with Ab against B220/CD11b and B220/F4/80, followed by immunofluorescence microscopy (×100). (E) WT and KO PECs were harvested, plated on glass slides by cytospin, and stained with Hema-3 reagent. Inset is a magnification of one representative monocytic/Mϕ cell. (F) F4/80+ pMϕ were treated with TNF-α (10 ng/ml), IFN-γ (500 U/ml), or a combination of both for 16 hr, followed by the measurement of NO in the supernatant. (G) Cells from (F) were analyzed by flow cytometry for cell-surface expression of I-A/E (MHC class II). Numerous experiments were done with the similar results.
Figure 3. Loss of Responsiveness of gp96 Null Cells to the Ligands of Both Intracellular and Cell-Surface TLRs
(A) PECs of the KO and WT mice were treated with LPS (100 ng/ml), Pam3CSK4 (1 μg/ml), R848 (1 μg/ml), flagellin (100 ng/ml), or IL-1β (100 ng/ml), followed by intracellular stain for TNF-α and IL-6. F4/80+ cells were gated on. At least five experiments were done with similar results. (B) BMDMs were incubated with LPS at the indicated concentrations, CpG or control oligonucleotide (GpC) at 1 μM for 4 hr. Supernatant was harvested; IL-6 and IL-12p40 were measured by ELISA in triplicates (mean ± SEM). *p < 0.05; **p < 0.01. The data are representative of three independent experiments. (C) Loss of TLR7 and TLR9 responses by gp96 KO Mϕ was not due to absence of TLR2 and TLR4. PEC cells were collected from WT, KO, and Tlr2–/–TLR4–/– mice, stimulated with 100 ng/ml LPS, 1 μg/ml Pam3Cysk4, and 1 μg/ml R848 for 5 hr, followed by intracellular staining for TNF-α. F4/80+ cells were gated on. For examining TLR9 responsiveness, PECs were primed with 500 U/ml IFN-γ for 20 hr, followed by stimulation with 1 μM CpG or GpC for another 5 hr. Shaded histograms represented staining for TNF-α without ligand stimulation. Two experiments were performed with the similar results. (D) IB of gp96 in WT, mutant pre-B cell, as well as mutant cell transfected with a gp96 expression vector. β-actin was blotted to serve as a loading control. Numbers represent M.W. markers. (E) Restoration of response of mutant pre-B cells to TLR ligands by stable expression of gp96. Various cells were stimulated with TLR ligands for 24 hr, followed by flow cytometry for GFP expression. Open and shaded histogram represents cells cultured in medium and stimulators, respectively. Numerous experiments were done with similar findings.
Figure 4. gp96 Is a Molecular Chaperone for TLR9
(A) The WT and gp96-deficient F4/80+ cells were analyzed for cell-surface expression of TLR2 and TLR4 (open histogram). Shaded histogram represents isotype control. (B) Comparative TLR mRNA level in WT and KO BMDM by Q-PCR. Number below represents number of threshold cycle (average of 2 duplicate runs). (C) IB analysis of TLR9, gp96, and β-actin in WT and KO cells. (D) Confocal analysis of TLR9 and gp96 expression in WT and KO BMDMs. Day +3 WT or KO BMDMs were transduced with a retroviral vector expressing TLR9-HA and enhanced GFP (EGFP) in the bicistronic fashion. 2 days later, cells were seeded and cultured on glass coverslips, fixed, permeablized, blocked, and double stained with mouse anti-HA Ab and rat anti-gp96 Ab, followed by goat anti-mouse Alexa Fluor 647 (TLR9, red) and goat anti-rat Alexa Fluor 488/Rhodomine Red (gp96, green) secondary Ab. Images were captured with a Zeiss LSM 510 Axiovert 100 confocal microscope equipped with an argon/krypton laser. (E) Confocal analysis of TLR9 expression. HEK293-TLR9 cells were cultured on glass coverslips, fixed, permeablized, and stained for gp96 (green) and TLR9 (red). The nucleus was counterstained with PI (blue). The merged images (gp96 and TLR9, light transmission and PI) were also shown. (F) gp96 is coprecipitated with TLR9. HEK293-TLR9 cells were immunoprecipitated with HA Ab or isotype control Ab, treated with nothing, PNGase F, or Endo H, followed by IB for gp96 and TLR9. (G) Total cell lysates of HEK293-TLR9 were immunoblotted for gp96, developed with substrates of either high sensitivity (HS) or low sensitivity (LS). (H) HEK293-TLR9 cells or WT BMDM were immunoprecipitated with 9G10, SPA851, or respective isotype control Ab, followed by SDA-PAGE and IB for gp96 and TLR9. (I) HEK293-TLR9 cells were treated with buffer only, tunicamycin, thapsigargin, or antimycin A. Cells were then immunoprecipitated for TLR9 followed by IB for gp96; the aliquot of the total cell lysates were immunoblotted for gp96, TLR9, and β-actin.
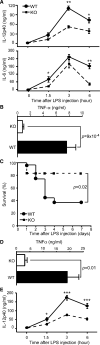
Figure 5. Ablation of gp96 in Macrophages Results in Attenuation of Endotoxin Shock
(A and B) 7-week-old KO mice (n = 5) or control littermates (n = 10) were administered i.p. with 0.5 mg LPS/mouse. Mice were bled at 0 (before LPS injection), 1.5, 3, and 6 hr after LPS injection. Serum IL-12p40 (A, top), IL-6 (A, bottom), and TNF-α (B) were quantified by ELISA. *p < 0.05; **p < 0.01. Two experiments were performed with similar findings. (C) Survival curve of KO (n = 12) or WT (n = 27) mice after i.p. injection of 0.5 mg LPS/mouse. (D and E) WT (n = 6) and KO (n = 5) mice were injected i.v. with 1 mg LPS/mouse, followed by measurement of serum TNF-α (D) and IL-12p40 (E).
Figure 6. LysMcre+/–Hsp90b1flox/– Mice Are Highly Susceptible to Listeria monocytogenes
(A) Weight loss of mice 3 days after i.p. injection of LmOVA at 2 × 106/mouse. Each symbol represents individual mouse. (B) Mice in (A) were bled at 24, 48, and 72 hr after LmOVA injection. Serum IL-12p40 was measured by ELISA. *p < 0.05; **p < 0.01. (C) _LmOVA_-infected mice were sacrificed 3 days after LmOVA injection. Bacteria load was measured. Symbols represent individual mice, and bars represent geometric mean of colony-forming unit (CFU)/organ. (D) H&E-stained sections from infected mice at day 3 after infection. Section from one representative mouse per group is shown (magnification ×200).
Comment in
- gp96 leads the way for toll-like receptors.
Harding CV. Harding CV. Immunity. 2007 Feb;26(2):141-3. doi: 10.1016/j.immuni.2007.02.003. Immunity. 2007. PMID: 17307701 Review.
Similar articles
- gp96 leads the way for toll-like receptors.
Harding CV. Harding CV. Immunity. 2007 Feb;26(2):141-3. doi: 10.1016/j.immuni.2007.02.003. Immunity. 2007. PMID: 17307701 Review. - Endoplasmic reticulum chaperone gp96 in macrophages is essential for protective immunity during Gram-negative pneumonia.
Anas AA, de Vos AF, Hoogendijk AJ, van Lieshout MH, van Heijst JW, Florquin S, Li Z, van 't Veer C, van der Poll T. Anas AA, et al. J Pathol. 2016 Jan;238(1):74-84. doi: 10.1002/path.4637. Epub 2015 Oct 19. J Pathol. 2016. PMID: 26365983 Free PMC article. - The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins.
Habich C, Baumgart K, Kolb H, Burkart V. Habich C, et al. J Immunol. 2002 Jan 15;168(2):569-76. doi: 10.4049/jimmunol.168.2.569. J Immunol. 2002. PMID: 11777948 - Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone.
Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li Y, Wu S, Li Y, Hao B, Bona R, Han D, Li Z. Liu B, et al. Nat Commun. 2010 Sep 21;1(6):79. doi: 10.1038/ncomms1070. Nat Commun. 2010. PMID: 20865800 Free PMC article. - Heat shock proteins as ligands of toll-like receptors.
Vabulas RM, Wagner H, Schild H. Vabulas RM, et al. Curr Top Microbiol Immunol. 2002;270:169-84. doi: 10.1007/978-3-642-59430-4_11. Curr Top Microbiol Immunol. 2002. PMID: 12467251 Review.
Cited by
- Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance.
Yu S, Gao N. Yu S, et al. Cell Mol Life Sci. 2015 Sep;72(17):3343-53. doi: 10.1007/s00018-015-1931-1. Epub 2015 May 23. Cell Mol Life Sci. 2015. PMID: 26001904 Free PMC article. Review. - Limitation of individual folding resources in the ER leads to outcomes distinct from the unfolded protein response.
Eletto D, Maganty A, Eletto D, Dersh D, Makarewich C, Biswas C, Paton JC, Paton AW, Doroudgar S, Glembotski CC, Argon Y. Eletto D, et al. J Cell Sci. 2012 Oct 15;125(Pt 20):4865-75. doi: 10.1242/jcs.108928. Epub 2012 Aug 1. J Cell Sci. 2012. PMID: 22854046 Free PMC article. - Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion.
Jensen S, Thomsen AR. Jensen S, et al. J Virol. 2012 Mar;86(6):2900-10. doi: 10.1128/JVI.05738-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258243 Free PMC article. Review. - Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94.
Ansa-Addo EA, Thaxton J, Hong F, Wu BX, Zhang Y, Fugle CW, Metelli A, Riesenberg B, Williams K, Gewirth DT, Chiosis G, Liu B, Li Z. Ansa-Addo EA, et al. Curr Top Med Chem. 2016;16(25):2765-78. doi: 10.2174/1568026616666160413141613. Curr Top Med Chem. 2016. PMID: 27072698 Free PMC article. Review. - GARP: a surface molecule of regulatory T cells that is involved in the regulatory function and TGF-β releasing.
Sun L, Jin H, Li H. Sun L, et al. Oncotarget. 2016 Jul 5;7(27):42826-42836. doi: 10.18632/oncotarget.8753. Oncotarget. 2016. PMID: 27095576 Free PMC article. Review.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. - PubMed
- Argon Y, Simen BB. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 1999;10:495–505. - PubMed
- Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 2006;7:49–56. - PubMed
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
- Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases