Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis - PubMed (original) (raw)
doi: 10.1016/j.cmet.2007.01.008.
Haihong Zong, Ji Li, Katsutaro Morino, Irene K Moore, Hannah J Yu, Zhen-Xiang Liu, Jianying Dong, Kirsty J Mustard, Simon A Hawley, Douglas Befroy, Marc Pypaert, D Grahame Hardie, Lawrence H Young, Gerald I Shulman
Affiliations
- PMID: 17276357
- PMCID: PMC1885964
- DOI: 10.1016/j.cmet.2007.01.008
Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis
Richard M Reznick et al. Cell Metab. 2007 Feb.
Abstract
Recent studies have demonstrated a strong relationship between aging-associated reductions in mitochondrial function, dysregulated intracellular lipid metabolism, and insulin resistance. Given the important role of the AMP-activated protein kinase (AMPK) in the regulation of fat oxidation and mitochondrial biogenesis, we examined AMPK activity in young and old rats and found that acute stimulation of AMPK-alpha(2) activity by 5'-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) and exercise was blunted in skeletal muscle of old rats. Furthermore, mitochondrial biogenesis in response to chronic activation of AMPK with beta-guanidinopropionic acid (beta-GPA) feeding was also diminished in old rats. These results suggest that aging-associated reductions in AMPK activity may be an important contributing factor in the reduced mitochondrial function and dysregulated intracellular lipid metabolism associated with aging.
Figures
Figure 1
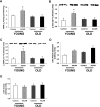
The Effect of AICAR Infusions on AMPK and LKB1 in Young and Old Rats (A) AMPK-α2 activity in the EDL muscle of young and old rats infused with either AICAR or saline (control). AMPK-α2 activity was increased by 44% in the young AICAR-treated rats compared to the young saline-treated rats. In contrast, there was no difference between the old AICAR-treated and saline-treated rats. (n = 7–10 in each group.) ∗p = 0.01. In this and all other figures, values are presented as means ± SEM. (B) p-ACC (Ser79) in the EDL muscle of young and old rats infused with either AICAR or saline (control). AICAR infusion resulted in a 120% increase in p-ACC (Ser79) in the young rats. In contrast, there was no effect of AICAR infusion on p-ACC (Ser79) in the old rats. (n = 4–6 in each group.) ∗p < 0.05. (C) p-AMPK (Thr172) in the EDL muscle of young and old rats infused with either AICAR or saline (control). AICAR infusion resulted in a 162% increase in p-AMPK (Thr172) in the young rats. In contrast, there was no effect of AICAR infusion on p-AMPK (Thr172) in the old rats. (n = 4–6 in each group.) ∗p < 0.05. (D) LKB1 protein expression in the EDL muscle of young and old rats infused with either AICAR or saline (control). LKB1 protein expression in EDL muscle of young and old rats did not differ significantly during aging or in response to AICAR infusion. (n = 4 in each group.) (E) LKB1 activity in the EDL muscle of young and old rats infused with either AICAR or saline (control). LKB1 activity in the EDL muscle of young and old rats did not differ significantly during aging or in response to AICAR infusion. (n = 4 in each group.)
Figure 2
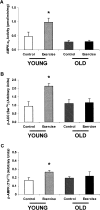
The Effect of Exercise on AMPK in Young and Old Rats (A) AMPK-α2 activity in the EDL muscle of exercising or sedentary young and old rats. AMPK-α2 activity was increased by 110% in the young exercising rats compared to the young sedentary rats. In contrast, there was no difference between the old exercising rats and old sedentary rats. (n = 8 in each group.) ∗p = 0.01. (B) p-ACC (Ser79) in the EDL muscle of exercising or sedentary young and old rats. Exercise resulted in a 127% increase in p-ACC (Ser79) in the young rats. In contrast, there was no effect of exercise on p-ACC (Ser79) in the old rats. (n = 4 in each group.) ∗p < 0.05. (C) p-AMPK (Thr172) in the EDL muscle of exercising or sedentary young and old rats. Exercise resulted in a 55% increase in p-AMPK (Thr172) in the young rats. In contrast, there was no effect of exercise on p-AMPK (Thr172) in the old rats. (n = 4 in each group.) ∗p < 0.05.
Figure 3
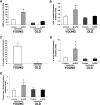
The Effect of β-GPA Feeding on AMPK and Mitochondrial Biogenesis in Young and Old Rats (A) AMPK-α2 activity in the EDL muscle of young and old rats fed either a β-GPA-supplemented diet or a control diet. The β-GPA-supplemented diet resulted in a 146% increase in AMPK-α2 activity in the young rats. In contrast, the β-GPA-supplemented diet had no effect on AMPK-α2 activity in the old rats. (n = 3–4 in each group.) ∗p < 0.01. (B) Pgc-1_α mRNA expression in the EDL muscle of young and old rats fed either a β-GPA-supplemented diet or a control diet. Pgc-1_α mRNA expression increased by 86% in the young β-GPA-fed rats. In contrast, there was no difference in Pgc-1_α mRNA expression in the old rats. (n = 5–8 in each group.) ∗p < 0.05. (C) Percent increase in mitochondrial density in the EDL muscle of young and old rats fed a β-GPA-supplemented diet compared to control rats. Mitochondrial density increased by 38% in the young β-GPA-fed rats. In contrast, the β-GPA-supplemented diet resulted in no difference in mitochondrial density in the old rats. (n = 4–5 in each group.) ∗p < 0.05. (D) δ_-Alas mRNA expression in the EDL muscle of young and old rats fed either a β-GPA-supplemented diet or a control diet. δ_-Alas mRNA expression increased by 289% in the young β-GPA-fed rats. In contrast, there was no difference in δ_-Alas mRNA expression in the old rats. (n = 3–8 in each group.) ∗p = 0.05. (E) Cytochrome c protein expression in the EDL muscle of young and old rats fed either a β-GPA-supplemented diet or a control diet. Cytochrome c protein expression increased by 76% in the young β-GPA-fed rats. In contrast, there was no difference in cytochrome c protein expression in the old rats. (n = 4–8 in each group.) ∗p < 0.05 .
Similar articles
- Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR.
Sakamoto K, Göransson O, Hardie DG, Alessi DR. Sakamoto K, et al. Am J Physiol Endocrinol Metab. 2004 Aug;287(2):E310-7. doi: 10.1152/ajpendo.00074.2004. Epub 2004 Apr 6. Am J Physiol Endocrinol Metab. 2004. PMID: 15068958 - Etoposide induces ATM-dependent mitochondrial biogenesis through AMPK activation.
Fu X, Wan S, Lyu YL, Liu LF, Qi H. Fu X, et al. PLoS One. 2008 Apr 23;3(4):e2009. doi: 10.1371/journal.pone.0002009. PLoS One. 2008. PMID: 18431490 Free PMC article. - AMP-activated protein kinase: possible target for treatment of type 2 diabetes.
Winder WW. Winder WW. Diabetes Technol Ther. 2000 Autumn;2(3):441-8. doi: 10.1089/15209150050194305. Diabetes Technol Ther. 2000. PMID: 11467346 Review. - Role of AMP-activated protein kinase in the control of appetite.
Kola B. Kola B. J Neuroendocrinol. 2008 Jul;20(7):942-51. doi: 10.1111/j.1365-2826.2008.01745.x. Epub 2008 Apr 28. J Neuroendocrinol. 2008. PMID: 18445126 Free PMC article. Review.
Cited by
- The Oldest of Old Male C57B/6J Mice Are Protected from Sarcopenic Obesity: The Possible Role of Skeletal Muscle Protein Kinase B Expression.
Reynolds TH 4th, Mills N, Hoyte D, Ehnstrom K, Arata A. Reynolds TH 4th, et al. Int J Mol Sci. 2024 Sep 24;25(19):10278. doi: 10.3390/ijms251910278. Int J Mol Sci. 2024. PMID: 39408607 Free PMC article. - The onset and the development of cardiometabolic aging: an insight into the underlying mechanisms.
Sarkar S, Prasanna VS, Das P, Suzuki H, Fujihara K, Kodama S, Sone H, Sreedhar R, Velayutham R, Watanabe K, Arumugam S. Sarkar S, et al. Front Pharmacol. 2024 Sep 26;15:1447890. doi: 10.3389/fphar.2024.1447890. eCollection 2024. Front Pharmacol. 2024. PMID: 39391689 Free PMC article. Review. - Mitochondrial network remodeling of the diabetic heart: implications to ischemia related cardiac dysfunction.
Rudokas MW, McKay M, Toksoy Z, Eisen JN, Bögner M, Young LH, Akar FG. Rudokas MW, et al. Cardiovasc Diabetol. 2024 Jul 18;23(1):261. doi: 10.1186/s12933-024-02357-1. Cardiovasc Diabetol. 2024. PMID: 39026280 Free PMC article. Review. - Mitochondrion quality control for longevity promotion.
Zhang Y, Chen S, Liu J. Zhang Y, et al. Metabol Open. 2023 Oct 21;22:100259. doi: 10.1016/j.metop.2023.100259. eCollection 2024 Jun. Metabol Open. 2023. PMID: 39011167 Free PMC article. No abstract available. - Therapeutic potential of berries in age-related neurological disorders.
Norouzkhani N, Afshari S, Sadatmadani SF, Mollaqasem MM, Mosadeghi S, Ghadri H, Fazlizade S, Alizadeh K, Akbari Javar P, Amiri H, Foroughi E, Ansari A, Mousazadeh K, Davany BA, Akhtari Kohnehshahri A, Alizadeh A, Dadkhah PA, Poudineh M. Norouzkhani N, et al. Front Pharmacol. 2024 May 9;15:1348127. doi: 10.3389/fphar.2024.1348127. eCollection 2024. Front Pharmacol. 2024. PMID: 38783949 Free PMC article. Review.
References
- Bergeron R., Ren J.M., Cadman K.S., Moore I.K., Perret P., Pypaert M., Young L.H., Semenkovich C.F., Shulman G.I. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1340–E1346. - PubMed
- Bolster D.R., Crozier S.J., Kimball S.R., Jefferson L.S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through downregulated mTOR signaling. J. Biol. Chem. 2002;277:23977–23980. - PubMed
- Corton J.M., Gillespie J.G., Hawley S.A., Hardie D.G. 5-aminoimidazole-4-carboxamide ribonucleoside: A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995;29:653–657. - PubMed
- Hardie D.G., Salt I.P., Davies S.P. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol. Biol. 2000;99:63–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL-63811/HL/NHLBI NIH HHS/United States
- U24 DK-59635/DK/NIDDK NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- P30 DK-45735/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- T32 GM007499/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- T32 GM-07499/GM/NIGMS NIH HHS/United States
- R01 DK-40936/DK/NIDDK NIH HHS/United States
- R01 HL063811/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical